Chemistry:Dichlormid
From HandWiki
| |
| Names | |
|---|---|
| Other names
N,N-diallyl-2,2-dichloroacetamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H11Cl2NO | |
| Molar mass | 208.08 g·mol−1 |
| Appearance | colorless oil |
| Melting point | 5.5 °C (41.9 °F; 278.6 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302, H315, H332 | |
| P261, P264, P270, P271, P280, P301+317Script error: No such module "Preview warning".Category:GHS errors, P302+352, P304+340, P317Script error: No such module "Preview warning".Category:GHS errors, P321, P330, P332+317Script error: No such module "Preview warning".Category:GHS errors, P362+364Script error: No such module "Preview warning".Category:GHS errors, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
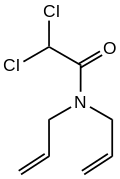
Dichlormid is an organic compound with the formula Cl
2CHCON(CH
2C=CH
2)
2. The compound can be classified as the amide of diallylamine and dichloroacetic acid. It is an herbicide safener for use with maize.[2]
References
- ↑ "Dichlormid" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/37829#section=Safety-and-Hazards.
- ↑ Riechers, Dean E.; Kreuz, Klaus; Zhang, Qin (2010). "Detoxification without Intoxication: Herbicide Safeners Activate Plant Defense Gene Expression". Plant Physiology 153 (1): 3–13. doi:10.1104/pp.110.153601. PMID 20237021.
 |

