Chemistry:Diallylamine
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
N-prop-2-enylprop-2-en-1-amine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2359 |
| |
| |
| Properties | |
| C6H11N | |
| Molar mass | 97.161 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.7874 g/cm3 |
| Melting point | −88 °C (−126 °F; 185 K) |
| Boiling point | 111 °C (232 °F; 384 K) |
| Hazards | |
| GHS pictograms |    
|
| GHS Signal word | Danger |
| H225, H302, H311, H314, H315, H319, H335, H412 | |
| P210, P233, P240, P241, P242, P243, P260, P261, P264, P264+265Script error: No such module "Preview warning".Category:GHS errors, P270, P271, P273, P280, P301+317Script error: No such module "Preview warning".Category:GHS errors, P301+330+331, P302+352, P302+361+354Script error: No such module "Preview warning".Category:GHS errors, P303+361+353, P304+340, P305+351+338, P305+354+338Script error: No such module "Preview warning".Category:GHS errors, P316Script error: No such module "Preview warning".Category:GHS errors, P317Script error: No such module "Preview warning".Category:GHS errors, P319Script error: No such module "Preview warning".Category:GHS errors | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
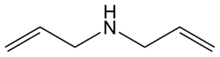
Diallylamine is the organic compound with the formula HN(CH2CH=CH2)2. It is a colorless liquid with an ammonia-like odor. It is multifunctional, featuring a secondary amine and two alkene groups. Diallylamine is used in the production of N,N-diallyldichloroacetamide (dichlormid) and N,N-diallyldimethylammonium chloride.[2]
Preparation
It is produced commercially by partial hydrogenation of acrylonitrile:[2]
- 2 NCCH=CH2 + 4 H2 → HN(CH2CH=CH2)2 + NH3
A laboratory route to diallylamine entails diallylation of calcium cyanamide followed by decyanation of the product.[3]
Related compounds
References
- ↑ "Diallylamine" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/31279#section=Safety-and-Hazards.
- ↑ 2.0 2.1 Eller, Karsten; Henkes, Erhard; Rossbacher, Roland; Höke, Hartmut (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_001.
- ↑ E. B. Vliet (1925). "Diallylamine". Organic Syntheses 5: 43. doi:10.15227/orgsyn.005.0043.
 |

