Chemistry:Diclofenac etalhyaluronate
From HandWiki
Short description: Medication
 | |
| Clinical data | |
|---|---|
| Trade names | Joycle |
| Other names | Diclofenac etalhyaluronate sodium (JAN); SI-613 |
| Legal status | |
| Legal status |
|
| Identifiers | |
| PubChem SID | |
| UNII | |
| KEGG | |
Diclofenac etalhyaluronate (INN, USAN; trade name Joycle) is an anti-inflammatory and joint function improving drug. In Japan it is approved for use in the treatment of knee osteoarthritis.[1][2]
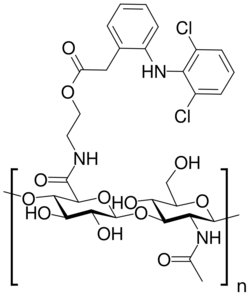
Chemically, diclofenac etalhyaluronate consists of the drug diclofenac, a nonsteroidal antiinflammatory drug, covalently linked to hyaluronic acid.[3][4] In the body, diclofenac is slowly cleaved and released, allowing diclofenac etalhyaluronate to function as a sustained-release form of diclofenac.
References
- ↑ "Diclofenac etalhyaluronate - Seikagku Corporation". Adis Insight. Springer Nature Switzerland AG. https://adisinsight.springer.com/drugs/800036809.
- ↑ "New Drug Approvals in Japan". KEGG. https://www.genome.jp/kegg/drug/br08318.html.
- ↑ "Effect of diclofenac etalhyaluronate (SI-613) on the production of high molecular weight sodium hyaluronate in human synoviocytes". BMC Musculoskelet Disord 20 (201): 201. 2019. doi:10.1186/s12891-019-2586-0. PMID 31077160.
- ↑ "Efficacy and Safety of Diclofenac-Hyaluronate Conjugate (Diclofenac Etalhyaluronate) for Knee Osteoarthritis: A Randomized Phase III Trial in Japan". Arthritis & Rheumatology (Hoboken, N.J.) 73 (9): 1646–1655. September 2021. doi:10.1002/art.41725. PMID 33749997.
 |

