Chemistry:Diclofenac
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Cataflam, Voltaren, Zipsor, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a689002 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, rectal, intramuscular, intravenous, topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | More than 99% |
| Metabolism | Liver, oxidative, primarily by CYP2C9, also by CYP2C8, CYP3A4, as well as conjugative by glucuronidation (UGT2B7) and sulfation;[7] no active metabolites exist |
| Onset of action | Within 4 hours (gel),[4] 30 min (non-gel)[5] |
| Elimination half-life | 1.2–2 h (35% of the drug enters enterohepatic recirculation) |
| Excretion | 35% bile, 65% urine[6] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
| Formula | C14H11Cl2NO2 |
| Molar mass | 296.15 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Diclofenac (pronounced /daɪˈkloʊfənæk/[1] or /dɪklɒˈfɛnæk/[8]), sold under the brand name Voltaren, among others, is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain and inflammatory diseases such as gout.[5] It is taken by mouth or rectally in a suppository, used by injection, or applied to the skin.[5][9] Improvements in pain last for as much as eight hours.[5] It is also available in combination with misoprostol in an effort to decrease stomach problems.[10]
Common side effects include abdominal pain, gastrointestinal bleeding, nausea, dizziness, headache, and swelling.[5] Serious side effects may include heart disease, stroke, kidney problems, and stomach ulceration.[10][5] Use is not recommended in the third trimester of pregnancy.[5] It is likely safe during breastfeeding.[10] Diclofenac is believed to work by decreasing the production of prostaglandins, like other drugs in this class.[11]
Diclofenac was patented in 1965 by J.R. Geigy AG;[12][13][verification needed] it came into medical use in the United States in 1988.[5] It is available as a generic medication.[5] In 2021, it was the 61st most commonly prescribed medication in the United States, with more than 11 million prescriptions.[14][15] It is available as its acid or in two salts, as either diclofenac sodium or potassium.[10] It is also widely used for livestock; such use was responsible for the Indian vulture crisis, during which in a few years 95% of the country's vulture population was killed, and in many countries agricultural use is now forbidden.[16][17][18][19]
Medical uses
Diclofenac is used to treat pain, inflammatory disorders, and dysmenorrhea.[20]
Pain
Inflammatory disorders may include arthritis, rheumatoid arthritis, polymyositis, dermatomyositis, osteoarthritis, dental pain, temporomandibular joint (TMJ) pain, spondylarthritis, ankylosing spondylitis, gout attacks,[21] and pain management in cases of kidney stones and gallstones. An additional indication is the treatment of acute migraines.[22] Diclofenac is used commonly to treat mild to moderate postoperative or post-traumatic pain, in particular when inflammation is also present.[21] It is also effective against endometriosis.
Diclofenac is also available in topical forms and has been found to be useful for osteoarthritis but not other types of long-term musculoskeletal pain.[23]
It may also help with actinic keratosis and with acute pain caused by minor strains, sprains and contusions (bruises).[24]
In many countries,[25] eye drops are sold to treat acute and chronic nonbacterial inflammation of the anterior part of the eyes (such as postoperative states). The eye drops have also been used to manage pain for traumatic corneal abrasion.[26]
Diclofenac is often used to treat chronic pain associated with cancer, especially if inflammation is present.[27] Use of diclofenac gel should not exceed 32 g (32,000 mg) in a day.[28]
-
Voltaren (diclofenac) 50 mg enteric coated tablets
-
Arthrotec (diclofenac and misoprostol) 50 mg tablets
-
Sintofarm (diclofenac) for suppository administration
-
150 gram tube diclofenac topical gel U.S. package generic
Contraindications
- Hypersensitivity to diclofenac
- History of allergic reactions (bronchospasm, shock, rhinitis, urticaria) to other NSAIDs, such as aspirin
- Third-trimester pregnancy
- Active stomach and/or duodenal ulceration or gastrointestinal bleeding
- Inflammatory bowel disease such as Crohn's disease or ulcerative colitis
- Severe congestive heart failure (NYHA III/IV)
- Pain management in the setting of coronary artery bypass graft (CABG) surgery
- Severe liver insufficiency (Child-Pugh Class C)
- Severe chronic kidney disease (creatinine clearance <30 ml/min)
- Caution in patients with pre-existing hepatic porphyria, as diclofenac may trigger attacks
- Caution in patients with severe, active bleeding such as cerebral hemorrhage
- NSAIDs in general should be avoided during dengue fever, as it induces (often severe) capillary leakage and subsequent heart failure.
- Caution in patients with fluid retention or heart failure
- Can lead to onset of new hypertension or worsening of pre-existing hypertension
- Can cause serious skin adverse events such as exfoliative dermatitis, Stevens–Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal[29]
Adverse effects
Diclofenac consumption has been associated with significantly increased vascular and coronary risk in a study including coxib, diclofenac, ibuprofen and naproxen.[30] Upper gastrointestinal complications were also reported.[30] Major adverse cardiovascular events (MACE) were increased by about a third by diclofenac, chiefly due to an increase in major coronary events.[30] Compared with placebo, of 1000 patients allocated to diclofenac for a year, three more had major vascular events, one of which was fatal.[30] Vascular death was increased significantly by diclofenac.[30]
In October 2020, the U.S. Food and Drug Administration (FDA) required the drug label to be updated for all nonsteroidal anti-inflammatory medications to describe the risk of kidney problems in unborn babies that result in low amniotic fluid.[31][32] They recommend avoiding NSAIDs in pregnant women at 20 weeks or later in pregnancy.[31][32]
Heart
In 2013, a study found major vascular events were increased by about a third by diclofenac, chiefly due to an increase in major coronary events.[30] Compared with placebo, of 1000 people allocated to diclofenac for a year, three more had major vascular events, one of which was fatal.[30] Vascular death was increased by diclofenac (1·65).[30]
Following the identification of increased risks of heart attacks with the selective COX-2 inhibitor rofecoxib in 2004, attention has focused on all the other members of the NSAIDs group, including diclofenac. Research results are mixed, with a meta-analysis of papers and reports up to April 2006 suggesting a relative increased rate of heart disease of 1.63 compared to nonusers.[33] Professor Peter Weissberg, medical director of the British Heart Foundation said, "However, the increased risk is small, and many patients with chronic debilitating pain may well feel that this small risk is worth taking to relieve their symptoms". Only aspirin was found not to increase the risk of heart disease; however, this is known to have a higher rate of gastric ulceration than diclofenac. In Britain the Medicines and Healthcare products Regulatory Agency (MHRA) said in June 2013 that the drug should not be used by people with serious underlying heart conditions – people who had had heart failure, heart disease or a stroke were advised to stop using it completely.[34] As of 15 January 2015, the MHRA announced that diclofenac will be reclassified as a prescription-only medicine (POM) due to the risk of cardiovascular adverse events.[35]
A subsequent large study of 74,838 Danish users of NSAIDs or coxibs found no additional cardiovascular risk from diclofenac use.[36] A very large study of 1,028,437 Danish users of various NSAIDs or coxibs found the "Use of the nonselective NSAID diclofenac and the selective cyclooxygenase-2 inhibitor rofecoxib was associated with an increased risk of cardiovascular death (odds ratio, 1.91; 95% confidence interval, 1.62 to 2.42; and odds ratio, 1.66; 95% confidence interval, 1.06 to 2.59, respectively), with a dose-dependent increase in risk."[37]
Diclofenac is similar in COX-2 selectivity to celecoxib.[38][contradictory]
Gastrointestinal
- Gastrointestinal complaints are most often noted. The development of ulceration and/or bleeding requires immediate termination of treatment with diclofenac. Most patients receive a gastro-protective drug as prophylaxis during long-term treatment (misoprostol, ranitidine 150 mg at bedtime or omeprazole 20 mg at bedtime).
Liver
- Liver damage occurs infrequently, and is usually reversible. Hepatitis may occur rarely without any warning symptoms and may be fatal. Patients with osteoarthritis more often develop symptomatic liver disease than patients with rheumatoid arthritis. Liver function should be monitored regularly during long-term treatment. If used for the short-term treatment of pain or fever, diclofenac has not been found more hepatotoxic than other NSAIDs.
- (As of December 2009), Endo, Novartis, and the US FDA notified healthcare professionals to add new warnings and precautions about the potential for elevation in liver function tests during treatment with all products containing diclofenac sodium.[39]
- Cases of drug-induced hepatotoxicity have been reported in the first month, but can occur at any time during treatment with diclofenac. Postmarketing surveillance has reported cases of severe hepatic reactions, including liver necrosis, jaundice, fulminant hepatitis with and without jaundice, and liver failure. Some of these reported cases resulted in fatalities or liver transplantation.
- Physicians should measure transaminases periodically in patients receiving long-term therapy with diclofenac. Based on clinical trial data and postmarketing experiences, transaminases should be monitored within 4 to 8 week after initiating treatment with diclofenac.
Kidney
- NSAIDs "are associated with adverse renal [kidney] effects caused by the reduction in synthesis of renal prostaglandins"[40] in sensitive persons or animal species, and potentially during long-term use in nonsensitive persons if resistance to side effects decreases with age. However, this side effect cannot be avoided merely by using a COX-2 selective inhibitor because, "Both isoforms of COX, COX-1 and COX-2, are expressed in the kidney... Consequently, the same precautions regarding renal risk that are followed for nonselective NSAIDs should be used when selective COX-2 inhibitors are administered."[40] However, diclofenac appears to have a different mechanism of renal toxicity.[citation needed]
- Studies in Spain showed diclofenac caused acute kidney failure in vultures when they ate the carcasses of animals that had recently been treated with it. Drug-sensitive species and individual humans are initially assumed to lack genes expressing specific drug detoxification enzymes.[41]
Mental health
- Mental health side effects have been reported. These symptoms are rare, but exist in significant enough numbers to include as potential side effects. These include depression, anxiety, irritability, nightmares, and psychotic reactions.[42]
Mechanism of action
As with most NSAIDs, the primary mechanism responsible for its anti-inflammatory, antipyretic and analgesic action is thought to be inhibition of prostaglandin synthesis through COX-inhibition. Diclofenac inhibits COX-1 and COX-2 with relative equipotency.[43]
The main target in inhibition of prostaglandin synthesis appears to be the transiently expressed prostaglandin-endoperoxide synthase-2 (PGES-2), also known as cycloxygenase-2 (COX-2). That is, diclofenac is partially selective for COX-2. It inhibits COX-2 approximately four times as much as COX-1.[44]
The drug may be bacteriostatic via inhibiting bacterial DNA synthesis.[45]
Diclofenac has a relatively high lipid solubility, making it one of the few NSAIDs that are able to enter the brain by crossing the blood-brain barrier.[46] As in the rest of the body, it is thought to exert its effect in the brain through inhibition of COX-2.[46] In addition, it may have effects inside the spinal cord.[47]
Diclofenac may be a unique member of the NSAIDs in other aspects. Some evidence indicates it inhibits the lipoxygenase pathways,[citation needed] thus reducing formation of leukotrienes (also pro-inflammatory autacoids). It also may inhibit phospholipase A2, which may be relevant to its mechanism of action. These additional actions may explain its high potency – it is the most potent NSAID on a broad basis.[48]
Marked differences exist among NSAIDs in their selective inhibition of the two subtypes of cyclooxygenase, COX-1 and COX-2.[49] Drug developers have focused on selective COX-2 inhibition, particularly as a way to minimize the gastrointestinal side effects of NSAIDs. In practice, use of some COX-2 inhibitors with their adverse effects has led to massive numbers of lawsuits alleging wrongful death by heart attack, yet other significantly COX-selective NSAIDs, such as diclofenac, have been well tolerated by most of the population.[citation needed]
Besides the COX-inhibition, a number of other molecular targets of diclofenac possibly contributing to its pain-relieving actions have recently been identified. These include:
- Blockage of voltage-dependent sodium channels (after activation of the channel, diclofenac inhibits its reactivation, also known as phase inhibition)[citation needed]
- Blockage of acid-sensing ion channels (ASICs)[50]
- Positive allosteric modulation of KCNQ- and BK-potassium channels (diclofenac opens these channels, leading to hyperpolarization of the cell membrane)[citation needed]
The duration of action (i.e., duration of pain relief) of a single dose is longer (6 to 8 h) than the drug’s 1.2–2 h half-life. This could be partly because it persists for over 11 hours in synovial fluids.[51]
Society and culture
History
In the United States, 1% diclofenac gel was approved by the FDA in 2007. It was approved as a prescription drug and was indicated for the relief of the pain of osteoarthritis of joints responsive to topical treatment; in particular, it was prescribed for the joints in the hands, knees and feet.[3] It has not been shown to work for strains, sprains, bruises or sports injuries.[3] It was intended for the temporary relief of joint pain due to the most common type of arthritis, osteoarthritis.[3] In February 2020, the gel became an over-the-counter drug, and the FDA granted the approval of the nonprescription product to GlaxoSmithKline plc.[3]
Formulations and brand names
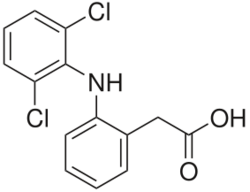
The name "diclofenac" derives from its chemical name: 2-(2,6-dichloranilino) phenylacetic acid. Diclofenac was first synthesized by Alfred Sallmann and Rudolf Pfister and introduced as Voltaren by Ciba-Geigy (now Novartis) in 1973. GlaxoSmithKline purchased the rights in 2015.[52]
Voltaren and Voltarol contain the sodium salt of diclofenac. In the United Kingdom, Voltarol can be supplied with either the sodium salt or the potassium salt, while Cataflam, sold in some other countries, is the potassium salt only. However, Voltarol Emulgel contains diclofenac diethylammonium 1.16%, being equivalent to 1% sodium salt. In 2016, Voltarol was one of the biggest selling branded over-the-counter medications sold in Great Britain, with sales of £39.3 million.[53]
In January 2015, diclofenac oral preparations were reclassified as prescription-only medicines in the UK. The topical preparations are available without prescription.[54]
Diclofenac formulations are available worldwide under many different brand names.[1]
Ecological effects
Use of diclofenac for animals is controversial due to toxicity when eaten by scavenging birds that eat dead animals;[16][17] the medication has been banned for veterinary use in several countries.[18][19]
Use of diclofenac in animals has been reported to have led to a sharp decline in the vulture population in the Indian subcontinent – a 95% decline by 2003[55] and a 99.9% decline by 2008. The mechanism is presumed to be renal failure;[56] however, toxicity may be due to direct inhibition of uric acid secretion in vultures.[57] Vultures eat the carcasses of livestock that have been administered veterinary diclofenac, and are poisoned by the accumulated chemical,[58] as vultures do not have a particular enzyme to break down diclofenac. At a meeting of the National Wildlife Board in March 2005, the Government of India announced it intended to phase out the veterinary use of diclofenac.[59] Meloxicam is a safer alternative to replace use of diclofenac.[60] It is more expensive than diclofenac, but the cost is dropping[when?] as more pharmaceutical companies are beginning to manufacture it.[citation needed]
Steppe eagles have the same vulnerability to diclofenac as vultures and may also fall victim to it.[61] Diclofenac has been shown also to harm freshwater fish species such as rainbow trout.[62][63][64][65] In contrast, New World vultures, such as the turkey vulture, can tolerate at least 100 times the level of diclofenac that is lethal to Gyps species.[66]
"The loss of tens of millions of vultures over the last decade has had major ecological consequences across the Indian subcontinent that pose a potential threat to human health. In many places, populations of feral dogs (Canis familiaris) have increased sharply from the disappearance of Gyps vultures as the main scavenger of wild and domestic ungulate carcasses. Associated with the rise in dog numbers is an increased risk of rabies"[60] and casualties of almost 50,000 people.[67] The Government of India cites this as one of the major consequences of a vulture species extinction.[59] A major shift in the transfer of corpse pathogens from vultures to feral dogs and rats could lead to a disease pandemic, causing millions of deaths in a crowded country like India, whereas vultures' digestive systems safely destroy many species of such pathogens. Vultures are long-lived and slow to breed. They start breeding only at the age of six and only 50% of young survive. Even if the government ban is fully implemented, it will take several years to revive the vulture population.[68]
The loss of vultures has had a social impact on the Indian Zoroastrian Parsi community, who traditionally use vultures to dispose of human corpses in Towers of Silence, but are now compelled to seek alternative methods of disposal.[60]
Despite the vulture crisis, diclofenac remains available in other countries including many in Europe.[69] It was controversially approved for veterinary use in Spain in 2013 and continues to be available, despite Spain being home to around 90% of the European vulture population and an independent simulation showing that the drug could reduce the population of vultures by 1–8% annually. Spain's medicine agency presented simulations suggesting that the number of deaths would be quite small.[41][70] A paper published in 2021 identified the first authenticated death of a vulture from diclofenac in Spain, a Cinereous Vulture.[71][17]
Diclofenac is on the European Union's watch list because it pollutes the Baltic Sea. When the substance enters freshwater, it has an environmental impact and is considered more difficult to remove in wastewater treatment plants than, for example, ibuprofen.[72] Harmful residues have been found in blue mussels and fish, among others, where it has been found to cause damage to internal organs such as the gills, kidneys and liver.[73]
Veterinary uses
Diclofenac is approved as a veterinary medication. It is used in the treatment of companion animals and livestock. In sheep, pigs, cattle and goats, it is used in the management of several bacterial diseases, including diarrhoea, enteritis, dysentery, foot rot and septicaemia.[74] In some bird species, diclofenac causes accumulation of uric acid crystals in organs, especially kidneys, triggering acute renal necrosis and visceral gout.[75] Vultures, among other carrion-eating birds, are known to scavenge deceased livestock. In South Asia in the 2000s, vulture populations were decimated after feeding on carcasses of livestock that had been treated with diclofenac.[41]
References
- ↑ 1.0 1.1 1.2 "Diclofenac". https://www.drugs.com/international/diclofenac.html.
- ↑ "Diclofenac Use During Pregnancy". 16 January 2000. https://www.drugs.com/pregnancy/diclofenac.html.
- ↑ 3.0 3.1 3.2 3.3 3.4 "FDA Approves Three Drugs for Nonprescription Use Through Rx-to-OTC Switch Process". 14 February 2020. http://www.fda.gov/news-events/press-announcements/fda-approves-three-drugs-nonprescription-use-through-rx-otc-switch-process.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "How Long Does It Take for Voltaren Gel to Work?" (in en-CA). https://www.youdrugstore.com/health/voltaren-gel-usage.html.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 5.8 "Diclofenac epolamine Monograph for Professionals". AHFS. https://www.drugs.com/monograph/diclofenac-epolamine.html.
- ↑ "Chapter 17 - Nonopioid analgesics: NSAIDs, COX-2 inhibitors, and acetaminophen" (in en). Essentials of Pain Medicine (Third ed.). W.B. Saunders. 1 January 2011. pp. 130–139. doi:10.1016/b978-1-4377-2242-0.00026-2. ISBN 978-1-4377-2242-0. https://www.sciencedirect.com/science/article/pii/B9781437722420000262. Retrieved 10 January 2023.
- ↑ "Diclofenac Oral Uses, Dosage, Side Effects And Composition". Medicine Reviews Agency. 23 August 2018. http://www.medicinereviews.ooo/2018/08/diclofenac-oral-uses-dosage-side.html.
- ↑ Mosby's Dictionary of Medicine, Nursing & Health Professions (tenth ed.). Elsevier. 2017. p. 536. ISBN 978-0-323-22205-1.
- ↑ "The use of Injectable Nonsteroidal Anti-Inflammatory Drugs in Local Accident & Emergency Practice". Hong Kong Journal of Emergency Medicine 9 (2): 65–71. 11 December 2017. doi:10.1177/102490790200900201.
- ↑ 10.0 10.1 10.2 10.3 British national formulary : BNF 74 (74 ed.). British Medical Association. 2017. pp. 1033–1035. ISBN 978-0-85711-298-9.
- ↑ Mosby's Drug Reference for Health Professions. Elsevier Health Sciences. 2017. p. 398. ISBN 978-0-323-56682-7. https://books.google.com/books?id=KOM2DwAAQBAJ&pg=PA398.
- ↑ Analogue-based drug discovery. Wiley-VCH. 2006. p. 517. ISBN 978-3-527-31257-3.
- ↑ Pfister R, Sallmann A, "Process for the production of new substituted phenylacetic acids", DE patent 1793592, issued 26 January 1978, assigned to Ciba Geigy AG
- ↑ "The Top 300 of 2021". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Diclofenac – Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Diclofenac.
- ↑ 16.0 16.1 "Avian scavengers and the threat from veterinary pharmaceuticals". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 369 (1656): 20130574. November 2014. doi:10.1098/rstb.2013.0574. PMID 25405963.
- ↑ 17.0 17.1 17.2 "The veterinary use of diclofenac and vulture conservation in Spain: Updated evidence and socio-ecological implications". The Science of the Total Environment 796: 148851. November 2021. doi:10.1016/j.scitotenv.2021.148851. PMID 34271379. Bibcode: 2021ScTEn.796n8851M.
- ↑ 18.0 18.1 ((European Medicines Agency)); ((Committee for Medicinal Products for Veterinary Use)), Opinion of the Committee pursuant to Article 30(3) of Regulation (EC) No 726/2004 on the risk to vultures and other necrophagous bird populations in the European Union in connection with the use of veterinary medicinal products containing the substance diclofenac, EMA/CVMP/761582/2014, https://www.ema.europa.eu/en/documents/other/opinion-committee-medicinal-products-veterinary-use-pursuant-article-303-regulation-ec-no-726/2004-risk-vultures-other-necrophagous-bird-populations-european-union-conne_en.pdf, retrieved 16 April 2022
- ↑ 19.0 19.1 "Rare European vultures being poisoned by livestock drug" (in en). The Guardian. 11 April 2021. https://www.theguardian.com/environment/2021/apr/11/rare-european-vultures-being-poisoned-by-livestock-drug. "...diclofenac has already been banned in India, Pakistan, Nepal and Bangladesh"
- ↑ "Diclofenac Epolamine". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/diclofenac-epolamine.html.
- ↑ 21.0 21.1 "Rufenal". Birzeit Pharmaceutical Company. http://www.bpc.ps/products/prod_desc/72.html.
- ↑ "Patient Site – Cambia (diclofenac potassium) for oral solution". http://www.cambiarx.com/.
- ↑ "Activity of diclofenac used alone and in combination with streptomycin against Mycobacterium tuberculosis in mice". International Journal of Antimicrobial Agents 30 (4): 336–340. October 2007. doi:10.1016/j.ijantimicag.2007.04.016. PMID 17644321.
- ↑ "Diclofenac (Topical Application Route) Description and Brand Names". Mayo Clinic. http://www.mayoclinic.com/health/drug-information/DR600545.
- ↑ "Naclof, oogdruppels 1 mg/ml". Laboratoires THEA. Netherlands: Netherlands Medicines Authority MEB. http://db.cbg-meb.nl/IB-teksten/h12800.pdf.
- ↑ "Topical non-steroidal anti-inflammatory drugs for analgesia in traumatic corneal abrasions". The Cochrane Database of Systematic Reviews 2017 (5): CD009781. May 2017. doi:10.1002/14651858.CD009781.pub2. PMID 28516471.
- ↑ "WHO's cancer pain ladder for adults". 27 November 2013. https://www.who.int/cancer/palliative/painladder/en/.
- ↑ "Topical NSAID therapy for musculoskeletal pain". Pain Medicine 11 (4): 535–549. April 2010. doi:10.1111/j.1526-4637.2010.00809.x. PMID 20210866.
- ↑ "Diclofenac Potassium". https://www.drugs.com/pro/diclofenac-potassium.html.
- ↑ 30.0 30.1 30.2 30.3 30.4 30.5 30.6 30.7 "Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials". Lancet 382 (9894): 769–779. August 2013. doi:10.1016/S0140-6736(13)60900-9. PMID 23726390.
- ↑ 31.0 31.1 "FDA Warns that Using a Type of Pain and Fever Medication in Second Half of Pregnancy Could Lead to Complications". U.S. Food and Drug Administration (FDA) (Press release). 15 October 2020. Archived from the original on 16 October 2020. Retrieved 15 October 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 32.0 32.1 "NSAIDs may cause rare kidney problems in unborn babies". 21 July 2017. https://www.fda.gov/drugs/drug-safety-and-availability/fda-recommends-avoiding-use-nsaids-pregnancy-20-weeks-or-later-because-they-can-result-low-amniotic.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials". BMJ 332 (7553): 1302–1308. June 2006. doi:10.1136/bmj.332.7553.1302. PMID 16740558.
- ↑ "Heart risk warning over painkiller". BBC News. 29 June 2013. https://www.bbc.co.uk/news/health-23109314.
- ↑ "Press release: Diclofenac tablets now only available as a prescription medicine". 14 January 2015. http://www.mhra.gov.uk/NewsCentre/Pressreleases/CON500341.
- ↑ "Cardiovascular outcomes in new users of coxibs and nonsteroidal antiinflammatory drugs: high-risk subgroups and time course of risk". Arthritis and Rheumatism 54 (5): 1378–1389. May 2006. doi:10.1002/art.21887. PMID 16645966.
- ↑ "Cause-specific cardiovascular risk associated with nonsteroidal antiinflammatory drugs among healthy individuals". Circulation: Cardiovascular Quality and Outcomes 3 (4): 395–405. July 2010. doi:10.1161/CIRCOUTCOMES.109.861104. PMID 20530789.
- ↑ "The coxibs, selective inhibitors of cyclooxygenase-2". The New England Journal of Medicine 345 (6): 433–442. August 2001. doi:10.1056/NEJM200108093450607. PMID 11496855.
- ↑ "Voltaren Gel (diclofenac sodium topical gel) 1% – Hepatic Effects Labeling Changes". 4 December 2009. https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm193047.htm.
- ↑ 40.0 40.1 "Renal effects of cyclooxygyenase-2-selective inhibitors". Journal of Pain and Symptom Management 23 (4 Suppl): S15–20; discussion S21–23. April 2002. doi:10.1016/S0885-3924(02)00370-6. PMID 11992745.
- ↑ 41.0 41.1 41.2 "Cattle drug threatens thousands of vultures". Nature. 29 April 2016. doi:10.1038/nature.2016.19839.
- ↑ "Diclofenac Side Effects". Drugs.com. https://www.drugs.com/sfx/diclofenac-side-effects.html.
- ↑ "Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase". Proceedings of the National Academy of Sciences of the United States of America 90 (24): 11693–11697. December 1993. doi:10.1073/pnas.90.24.11693. PMID 8265610. Bibcode: 1993PNAS...9011693M.
- ↑ Alfaro, Robert; Davis, Donald, eds (15 January 2024). "Diclofenac". https://www.ncbi.nlm.nih.gov/books/NBK557879/.
- ↑ "The anti-bacterial action of diclofenac shown by inhibition of DNA synthesis". International Journal of Antimicrobial Agents 14 (3): 249–251. April 2000. doi:10.1016/S0924-8579(99)00159-4. PMID 10773497.
- ↑ 46.0 46.1 "Diclofenac: update on tolerableness and spinal anti-inflammatory action". Minerva Medica 105 (4): 313–318. August 2014. PMID 25078485. https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2014N04A0313. Retrieved 23 April 2023.
- ↑ "Spinal antinflammatory action of Diclofenac". Minerva Medica 107 (3): 167–172. June 2016. PMID 27014880. https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2016N03A0167. Retrieved 23 April 2023.
- ↑ "Pharmacology of diclofenac sodium". The American Journal of Medicine 80 (4B): 34–38. April 1986. doi:10.1016/0002-9343(86)90077-x. PMID 3085490.
- ↑ "Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs". The American Journal of Medicine 104 (5): 413–421. May 1998. doi:10.1016/S0002-9343(98)00091-6. PMID 9626023.
- ↑ "Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors". The Journal of Neuroscience 21 (20): 8026–8033. October 2001. doi:10.1523/JNEUROSCI.21-20-08026.2001. PMID 11588175.
- ↑ "Plasma and synovial fluid concentrations of diclofenac sodium and its major hydroxylated metabolites during long-term treatment of rheumatoid arthritis". European Journal of Clinical Pharmacology 25 (3): 389–394. 1983. doi:10.1007/BF01037953. PMID 6628528.
- ↑ "Advances in NSAID development: evolution of diclofenac products using pharmaceutical technology". Drugs 75 (8): 859–877. May 2015. doi:10.1007/s40265-015-0392-z. PMID 25963327.
- ↑ "A breakdown of the over-the-counter medicines market in Britain in 2016". The Pharmaceutical Journal. 28 April 2017. https://pharmaceutical-journal.com/article/infographics/a-breakdown-of-the-over-the-counter-medicines-market-in-britain-in-2016.
- ↑ "Oral diclofenac presentations with legal status 'P' – reclassified to POM". https://www.gov.uk/drug-device-alerts/drug-alert-oral-diclofenac-presentations-with-legal-status-p-reclassified-to-pom.
- ↑ "Diclofenac residues as the cause of vulture population decline in Pakistan". Nature 427 (6975): 630–633. February 2004. doi:10.1038/nature02317. PMID 14745453. Bibcode: 2004Natur.427..630O.
- ↑ "Toxicity of diclofenac to Gyps vultures". Biology Letters 2 (2): 279–282. June 2006. doi:10.1098/rsbl.2005.0425. PMID 17148382.
- ↑ "Diclofenac toxicity in Gyps vulture is associated with decreased uric acid excretion and not renal portal vasoconstriction". Comparative Biochemistry and Physiology. Toxicology & Pharmacology 149 (3): 269–274. April 2009. doi:10.1016/j.cbpc.2008.07.014. PMID 18727958.
- ↑ "Vet drug 'killing Asian vultures'". BBC News. 28 February 2004. http://news.bbc.co.uk/2/hi/science/nature/3437583.stm.
- ↑ 59.0 59.1 "Saving the Vultures from Extinction" (Press release). Press Information Bureau, Government of India. 16 May 2005. Archived from the original on 20 December 2005. Retrieved 12 May 2006.
- ↑ 60.0 60.1 60.2 "Removing the threat of diclofenac to critically endangered Asian vultures". PLOS Biology 4 (3): e66. March 2006. doi:10.1371/journal.pbio.0040066. PMID 16435886.
- ↑ "Eagles fall prey to vulture-killing chemical". Pune Mirror. 28 May 2014. http://www.punemirror.in/pune/others/Eagles-fall-prey-to-vulture-killing-chemical/articleshow/35639257.cms.
- ↑ "Toxic effects of the non-steroidal anti-inflammatory drug diclofenac. Part I: histopathological alterations and bioaccumulation in rainbow trout". Aquatic Toxicology 68 (2): 141–150. June 2004. doi:10.1016/j.aquatox.2004.03.014. PMID 15145224. Bibcode: 2004AqTox..68..141S.
- ↑ "Toxic effects of the non-steroidal anti-inflammatory drug diclofenac. Part II: cytological effects in liver, kidney, gills and intestine of rainbow trout (Oncorhynchus mykiss)". Aquatic Toxicology 68 (2): 151–166. June 2004. doi:10.1016/j.aquatox.2004.03.015. PMID 15145225. Bibcode: 2004AqTox..68..151T.
- ↑ "Subletale Wirkungen von Arzneimitteln bei aquatischen Organismen" (in de). Texte 29 (5): 217–226. 2005. https://www.umweltbundesamt.de/sites/default/files/medien/publikation/long/2976.pdf. Retrieved 23 April 2023.
- ↑ "Ultrastructural effects of pharmaceuticals (carbamazepine, clofibric acid, metoprolol, diclofenac) in rainbow trout (Oncorhynchus mykiss) and common carp (Cyprinus carpio)". Analytical and Bioanalytical Chemistry 387 (4): 1405–1416. February 2007. doi:10.1007/s00216-006-1033-x. PMID 17216161.
- ↑ "Apparent tolerance of turkey vultures (Cathartes aura) to the non-steroidal anti-inflammatory drug diclofenac". Environmental Toxicology and Chemistry 27 (11): 2341–2345. November 2008. doi:10.1897/08-123.1. PMID 18476752. http://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1977&context=usgsstaffpub. Retrieved 15 July 2019.
- ↑ "Rabies tragedy follows loss of India's vultures". New Scientist. 6 August 2008. https://www.newscientist.com/article/mg19926684-400-rabies-tragedy-follows-loss-of-indias-vultures/.
- ↑ "'Decline in vulture population has given rise to diseases': Dr Vibhu Prakash". The Indian Express. 29 August 2016. https://indianexpress.com/article/lifestyle/health/decline-in-vulture-population-has-given-rise-to-diseases-dr-vibhu-prakash-3001298/.
- ↑ "E-010588/2015: answer given by Mr Andriukaitis on behalf of the Commission". http://www.europarl.europa.eu/sides/getAllAnswers.do?reference=E-2015-010588&language=EN.
- ↑ "Vulture killing drug now available on EU market". International BirdLife. http://www.birdlife.org/europe-and-central-asia/news/vulture-killing-drug-now-available-eu-market.
- ↑ "First evidence of a vulture killed by veterinary diclofenac in Spain – will the Spanish government and the EU act after this smoking gun?". Vulture Conservation Foundation. 7 April 2021. https://www.4vultures.org/first-evidence-of-a-vulture-killed-by-veterinary-diclofenac-in-spain.
- ↑ "Val av smärtstillande påverkar miljön" (in sv-SE). 4 March 2010. https://www.lakemedelsvarlden.se/val-av-smartstillande-paverkar-miljon/.
- ↑ "Itämeren kalat häiriintyvät lääkeaineista – Teollisuudella paineita kehittää eettisempiä pillereitä" (in fi). 10 September 2014. https://yle.fi/a/3-7455669.
- ↑ "Diclofenac Sodium Injection: Product Information". AdvaCare Pharma USA. 2018. https://www.advacarepharma.com/en/amp/veterinary/diclofenac-sodium-injection.
- ↑ "Toxicological effects of diclofenac in four avian species". Avian Pathology 37 (3): 315–321. June 2008. doi:10.1080/03079450802056439. PMID 18568659.
External links
 |