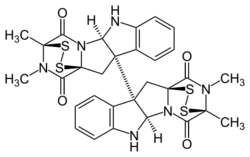
Chemistry:Dideoxyverticillin A
| |
| Names | |
|---|---|
| Other names
11,11’-dideoxyverticillin A, 11,11’-dideoxyverticillin, CHEMBL2172426
| |
| Identifiers | |
| ChemSpider | |
PubChem CID
|
|
| Properties | |
| C30H28N6O4S4 | |
| Molar mass | 664.84 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dideoxyverticillin A (+)-11,11’-dideoxyverticillin A is a complex epipolythiodioxopiperazine [1] initially isolated from the marine fungus Penicillium sp. in 1999 [2] has also been found in the marine fungus (Bionectriaceae),[3] and belongs to a class of naturally occurring 2,5-diketopiperazines.[4] Dideoxyverticillin A potently inhibits the tyrosine kinase activity of the epidermal growth factor receptor (median inhibitory concentration = 0.14 nM), exhibits antiangiogenic activity, and has efficacy against several cancer cell lines.[4] Its reported anticancer mechanism is that it acts as a farnesyl transferase inhibitor. Dozens of semi-synthetic anticancer compounds have been made from Dideoxyverticillin A. Dimeric derivatives are reported to have better anticancer activity.[5] The enantioselective first total synthesis of (+)-11,11’-dideoxyverticillin A, the structure of which contains many sterically congested, contiguous stereogenic centers as well as acid- and base-labile and redox-sensitive functionality, was biosynthetically inspired and achieved with high levels of chemical sophistication.[6]
References
- ↑ "The epipolythiodioxopiperazine (ETP) class of fungal toxins: distribution, mode of action, functions and biosynthesis". Microbiology 151 (4): 1021–1032. April 2005. doi:10.1099/mic.0.27847-0. PMID 15817772.
- ↑ "New cytotoxic epidithiodioxopiperazines related to verticillin A from a marine isolate of the fungus Penicillium". Natural Product Letters 13 (3): 213–222. May 1999. doi:10.1080/10575639908048788.
- ↑ "Cytotoxic epipolythiodioxopiperazine alkaloids from filamentous fungi of the Bionectriaceae". The Journal of Antibiotics 65 (11): 559–564. November 2012. doi:10.1038/ja.2012.69. PMID 22968289.
- ↑ 4.0 4.1 Borthwick AD (May 2012). "2,5-Diketopiperazines: Synthesis, Reactions, Medicinal Chemistry, and Bioactive Natural Products". Chemical Reviews 112 (7): 3641–3716. doi:10.1021/cr200398y. PMID 22575049.
- ↑ "Synthesis and anticancer activity of epipolythiodiketopiperazine alkaloids". Chemical Science 4 (4): 1646–1657. 2013. doi:10.1039/C3SC50174D. PMID 23914293.
- ↑ "Total synthesis of (+)-11,11'-dideoxyverticillin A". Science 324 (5924): 238–241. April 2009. doi:10.1126/science.1170777. PMID 19359584.
External links
- MIT News - 11,11’-Dideoxyverticillin
- "11-脱氧轮枝菌素A引起前列腺癌PC3M细胞Caspase依赖的凋亡" (in Chinese). Sheng Wu Gong Cheng Xue Bao 28 (1): 96–103. January 2012. PMID 22667113. http://d.wanfangdata.com.cn/Periodical_swgcxb201201011.aspx.
 |
