Chemistry:Digallic acid
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
3,4-Dihydroxy-5-[(3,4,5-trihydroxybenzoyl)oxy]benzoic acid | |
| Other names
Digallate
3,4-dihydroxy-5-(3,4,5-trihydroxybenzoyloxy)benzoate m-digallic acid Digalloyl ester | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
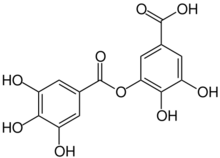
| C14H10O9 | |
| Molar mass | 322.225 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Digallic acid is a polyphenolic compound found in Pistacia lentiscus.[1] Digallic acid is also present in the molecule of tannic acid.[2] Digalloyl esters involve either -meta, or -para depside bonds.[3]
Tannase is an enzyme that uses digallate to produce gallic acid. This enzyme can also be used to produce digallic acid from gallotannins.[4]
References
- ↑ Bhouri, W.; Derbel, S.; Skandrani, I.; Boubaker, J.; Bouhlel, I.; Sghaier, M. B.; Kilani, S.; Mariotte, A. M. et al. (2010). "Study of genotoxic, antigenotoxic and antioxidant activities of the digallic acid isolated from Pistacia lentiscus fruits". Toxicology in Vitro 24 (2): 509–515. doi:10.1016/j.tiv.2009.06.024. PMID 19563883.
- ↑ Delahaye, P.; Verzele, M. (1983). "Analysis of gallic, digallic and trigallic acids in tannic acids by high-performance liquid chromatography". Journal of Chromatography A 265: 363–367. doi:10.1016/S0021-9673(01)96734-2.
- ↑ Ann E. Hagerman. "The Tannin Handbook". Miami University. http://www.users.miamioh.edu/hagermae/.
- ↑ Nierenstein, M. (1932). "A biological synthesis of m-digallic acid". The Biochemical Journal 26 (4): 1093–1094. doi:10.1042/bj0261093. PMID 16744910.
 |
