Chemistry:Diisopropylbenzenes
From HandWiki
Short description: Hydrocarbon compound; benzene ring with two isopropyl groups
In organic chemistry, the diisopropylbenzenes constitute a group of aromatic hydrocarbons, whose chemical structure consists of a benzene ring (C
6H
6) with two isopropyl groups (–CH(CH
3)
2) as substituents. Through their different arrangement, they form three structural isomers with the molecular formula C
12H
18.
| Diisopropylbenzenes | |||
| Systematic name | 1,2-Diisopropylbenzene | 1,3-Diisopropylbenzene | 1,4-Diisopropylbenzene |
| Common name | o-Diisopropylbenzene | m-Diisopropylbenzene | p-Diisopropylbenzene |
| Chemical structure | 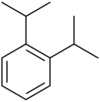
|
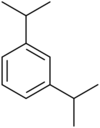
|
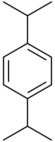
|
| CAS Number | 577-55-9 | 99-62-7 | 100-18-5 |
| PubChem | CID 11345 from PubChem | CID 7450 from PubChem | CID 7486 from PubChem |
| Chemical formula | C 12H 18 | ||
| Molar mass | 162.28 g/mol | ||
| State of matter | Liquid | ||
| Melting point | −57 °C[1] | −63 °C[2] | −17 °C[3] |
| Boiling point | 205 °C[1] | 203 °C[2] | 210 °C[3] |
| Solubility | Very slightly soluble in water[1] | 0.072 mg·l−1 in water (25 °C)[2] | Practically insoluble in water[3] |
References
- ↑ 1.0 1.1 1.2 Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ↑ 2.0 2.1 2.2 Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ↑ 3.0 3.1 3.2 Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health

