Chemistry:Diphenylcyclopropenone
| |
| Names | |
|---|---|
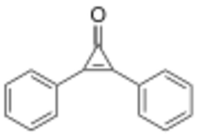
| Preferred IUPAC name
2,3-Diphenylcycloprop-2-en-1-one | |
| Other names
Diphencyprone, DPCP, DPC
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H10O | |
| Molar mass | 206.244 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Diphenylcyclopropenone (diphencyprone) is a topically administered experimental drug intended for treating alopecia areata and alopecia totalis.[1] Topical immunotherapy using diphenylcyclopropenone may also be an effective treatment option for recalcitrant warts.[2] It is not approved by either the Food and Drug Administration or the European Medicines Agency.[3]
Mechanism of action
Diphenylcyclopropenone triggers an immune response that is thought to oppose the action of the autoreactive cells that otherwise cause hair loss.[4] One hypothesis is that in response to DPCP treatment, the body will attempt to downregulate inflammation through a variety of pathways, resulting in a downregulation of the autoimmune response at the hair follicle. This autoinflammatory reaction would otherwise destroy body's hair follicles.[3]
Studies
A study of 41 alopecia areata patients showed significant hair regrowth in 40% at 6 months, being sustained in two thirds of these after a 12-month-follow up-period.[5]
In a 2002 study for the treatment of warts, the responders consisted of 135 individuals (87.7%) that had complete clearance of warts. Reported adverse effects were local and included with pruritus (itching) (15.6%), with blistering (7.1%), and with eczematous reactions (eczema)(14.2%). The majority of the patients tolerated the treatment very well. One patient developed local impetigo (minor infection). Patients had an average of 5 treatments over a 6-month period.[2]
Chemical properties
The chemical properties of diphenylcyclopropenone are dominated by the strong polarization of the carbonyl group, which gives a partial positive charge with aromatic stabilization on the cyclopropene ring and a partial negative charge on oxygen. Furthermore, the phenyl groups stabilize the partial positive charge in the ring through resonance.
Diphenylcyclopropenone reacts with electrophilic chlorinating agents, including oxalyl chloride, thionyl chloride and phosphorus pentachloride, to give 3,3-dichloro-1,2-diphenylcyclopropene, which is a reagent for the activation of carboxylic acids.[6]
See also
References
- ↑ "Topical immunotherapy in alopecia areata". International Journal of Trichology 2 (1): 36–9. January 2010. doi:10.4103/0974-7753.66911. PMID 21188022.
- ↑ 2.0 2.1 "The use of diphenylcyclopropenone in the treatment of recalcitrant warts". Journal of Cutaneous Medicine and Surgery 6 (3): 214–7. 2002. doi:10.1007/s10227-001-0050-9. PMID 11951129.
- ↑ 3.0 3.1 "Diphencyprone Treatment of Alopecia Areata: Postulated Mechanism of Action and Prospects for Therapeutic Synergy with RNA Interference". The Journal of Investigative Dermatology. Symposium Proceedings 17 (2): 16–8. November 2015. doi:10.1038/jidsymp.2015.33. PMID 26551938.
- ↑ Public summary of positive opinion for orphan designation of diphenylcyclopropenone for the treatment of alopecia totalis, European Medicines Agency. Document Date: London, 23 April 2009. Doc.Ref.:EMEA/COMP/428277/2006
- ↑ "Topical immunotherapy with diphenylcyclopropenone in the treatment of chronic extensive alopecia areata". Clinical and Experimental Dermatology 32 (1): 48–51. January 2007. doi:10.1111/j.1365-2230.2006.02256.x. PMID 17004987.
- ↑ Bennett, Clay S. (2012). "3,3-Dichloro-1,2-diphenylcyclopropene" (in en). Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289x.rn01514. ISBN 978-0471936237.
 |

