Chemistry:1,4-Butanedithiol
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C4H10S2 | |
| Molar mass | 122.24 g·mol−1 |
| Appearance | colorless liquid |
| Melting point | −53.9 °C (−65.0 °F; 219.2 K) |
| Boiling point | 195.5 °C (383.9 °F; 468.6 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| HH315Script error: No such module "Preview warning".Category:GHS errors, HH319Script error: No such module "Preview warning".Category:GHS errors, HH335Script error: No such module "Preview warning".Category:GHS errors | |
| PP261Script error: No such module "Preview warning".Category:GHS errors, PP264Script error: No such module "Preview warning".Category:GHS errors, PP264+P265Script error: No such module "Preview warning".Category:GHS errors, PP271Script error: No such module "Preview warning".Category:GHS errors, PP280Script error: No such module "Preview warning".Category:GHS errors, PP302+P352Script error: No such module "Preview warning".Category:GHS errors, PP304+P340Script error: No such module "Preview warning".Category:GHS errors, PP305+P351+P338Script error: No such module "Preview warning".Category:GHS errors, PP319Script error: No such module "Preview warning".Category:GHS errors, PP321Script error: No such module "Preview warning".Category:GHS errors, PP332+P317Script error: No such module "Preview warning".Category:GHS errors, PP337+P317Script error: No such module "Preview warning".Category:GHS errors, PP362+P364Script error: No such module "Preview warning".Category:GHS errors, PP403+P233Script error: No such module "Preview warning".Category:GHS errors, PP405Script error: No such module "Preview warning".Category:GHS errors, PP501Script error: No such module "Preview warning".Category:GHS errors | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
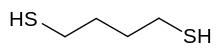
1,4-Butanedithiol is an organosulfur compound with the formula HSCH
2CH
2CH
2CH
2SH. It is a malodorous, colorless liquid that is highly soluble in organic solvents. The compound has found applications in biodegradable polymers.[2]
Reactions
Alkylation with geminal dihalides gives 1,3-dithiepanes. Oxidation gives the cyclic disulfide 1,2-dithiane:[3]
- HSCH
2CH
2CH
2CH
2SH + O → S
2(CH
2)
4 + H
2O
It forms self-assembled monolayers on gold.[4]
It is also used in polyadditions along with 1,4-butanediol to form sulfur-containing polyester and polyurethanes containing diisocyanate.[5][6][7] Several of these polymers are considered biodegradable and many of their components are sourced from non-petroleum oils.[8]
Related compounds
References
- ↑ "1,4-Butanedithiol" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/79148#section=Safety-and-Hazards.
- ↑ Türünç, Oĝuz; Meier, Michael A. R. (2011). "Thiol-ene vs. ADMET: a complementary approach to fatty acid-based biodegradable polymers" (in en). Green Chemistry 13 (2): 314. doi:10.1039/c0gc00773k. ISSN 1463-9262. http://xlink.rsc.org/?DOI=c0gc00773k.
- ↑ Oba, Makoto; Tanaka, Kazuhito; Nishiyama, Kozaburo; Ando, Wataru (2011). "Aerobic Oxidation of Thiols to Disulfides Catalyzed by Diaryl Tellurides under Photosensitized Conditions". The Journal of Organic Chemistry 76 (10): 4173–4177. doi:10.1021/jo200496r. PMID 21480642.
- ↑ Park, Jong-Won; Shumaker-Parry, Jennifer S. (2014). "Structural Study of Citrate Layers on Gold Nanoparticles: Role of Intermolecular Interactions in Stabilizing Nanoparticles". Journal of the American Chemical Society 136 (5): 1907–1921. doi:10.1021/ja4097384. PMID 24422457.
- ↑ Kojio, Ken; Nozaki, Shuhei; Takahara, Atsushi; Yamasaki, Satoshi (2020). "Influence of chemical structure of hard segments on physical properties of polyurethane elastomers: a review" (in en). Journal of Polymer Research 27 (6). doi:10.1007/s10965-020-02090-9. ISSN 1022-9760. https://link.springer.com/10.1007/s10965-020-02090-9.
- ↑ Sakhno, T. V.; Sakhno, Yu. E.; Kuchmiy, S. Ya. (2023). "Clusteroluminescence of Unconjugated Polymers: A Review" (in en). Theoretical and Experimental Chemistry 59 (2): 75–106. doi:10.1007/s11237-023-09768-3. ISSN 0040-5760. https://link.springer.com/10.1007/s11237-023-09768-3.
- ↑ Manzano, Verónica E.; Kolender, Adriana A.; Varela, Oscar (2017), Goyanes, Silvia Nair; D’Accorso, Norma Beatriz, eds., "Synthesis and Applications of Carbohydrate-Based Polyurethanes" (in en), Industrial Applications of Renewable Biomass Products (Cham: Springer International Publishing): pp. 1–43, doi:10.1007/978-3-319-61288-1_1, ISBN 978-3-319-61287-4, http://link.springer.com/10.1007/978-3-319-61288-1_1, retrieved 2023-12-05
- ↑ Kreye, Oliver; Tóth, Tommy; Meier, Michael A. R. (2011-09-01). "Copolymers derived from rapeseed derivatives via ADMET and thiol-ene addition". European Polymer Journal 47 (9): 1804–1816. doi:10.1016/j.eurpolymj.2011.06.012. ISSN 0014-3057. https://www.sciencedirect.com/science/article/pii/S0014305711002424.
 |
