Chemistry:Dodecahydroxycyclohexane
| |
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Cyclohexanedodecol | |
| Other names
Dodecahydroxycyclohexane
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| (C(OH) 2) 6 | |
| Molar mass | 276.150 g·mol−1 |
| Appearance | Colourless crystals (dihydrate) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
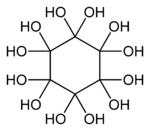
Dodecahydroxycyclohexane is an organic compound with molecular formula C
6O
12H
12 or C
6(OH)
12 or (C(OH)
2)
6. It is a sixfold geminal diol with a cyclohexane backbone and can be regarded as a sixfold hydrate of cyclohexanehexone (C
6O
6).
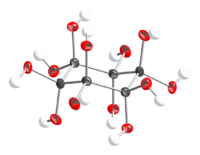
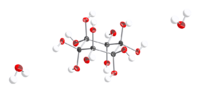
Dihydrate
The dihydrate C
6O
12H
12 · 2H2O can be crystallized from methanol as colorless plates or prisms, that decomposes at about 100 °C.[1]
This compound was synthesized by J. Lerch[2] in 1862 by oxidation of benzenehexol C
6(OH)
6 or tetrahydroxy-p-benzoquinone C
6(OH)
4O
2 and characterized by R. Nietzki and others in 1885,[3] although the product was for a long time assumed to be hexaketocyclohexane with water of crystallization (C
6O
6 · 8H2O).
Indeed, this product is still commonly marketed as cyclohexanehexone octahydrate, hexaketocyclohexane octahydrate, triquinoyl octahydrate and similar names. Its true nature was suspected since the 1950s or earlier,[4] but was confirmed by X-ray diffraction analysis only in 2005.[5]
See also
References
- ↑ Alexander J. Fatiadi; Horace S. Isbell; William F. Sager (March–April 1963). "Cyclic Polyhydroxy Ketones. I. Oxidation Products of Hexahydroxybenzene (Benzenehexol)". Journal of Research of the National Bureau of Standards Section A 67A (2): 153–162. doi:10.6028/jres.067A.015. PMID 31580622. PMC 6640573. http://nvl.nist.gov/pub/nistpubs/jres/067/2/V67.N02.A06.pdf. Retrieved 2009-03-22.
- ↑ Jos. Ud. Lerch (1862). "Ueber Kohlenoxydkalium und die aus demselben darstellbaren Säuren". Journal für Praktische Chemie 87 (1): 427–469. doi:10.1002/prac.18620870146. https://zenodo.org/record/1427832.
- ↑ R. Nietzki, Th. Benckiser (1885). "Ueber Hexaoxybenzolderivate und ihre Beziehungen zur Krokonsäure und Rhodizonsäure". Berichte der Deutschen Chemischen Gesellschaft 18 (1): 499–515. doi:10.1002/cber.188501801110. https://zenodo.org/record/1425379.
- ↑ Willis B. Person and Dale G. Williams (1957). "Infrared spectra and the structures of leuconic acid and triquinoyl". J. Phys. Chem. 61 (7): 1017–1018. doi:10.1021/j150553a047.
- ↑ Thomas M. Klapötke; Kurt Polborn; Jan J. Weigand (March 2005). "Dodecahydroxycyclohexane dihydrate". Acta Crystallographica E. http://journals.iucr.org/e/issues/2005/05/00/ob6498/ob6498.pdf.
 |

