Chemistry:Cyclohexanehexone
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
cyclohexane-1,2,3,4,5,6-hexone
| |||
| Other names
hexaketocyclohexane, triquinoyl
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C6O6 | |||
| Molar mass | 168.060 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
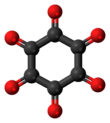
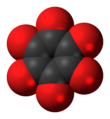
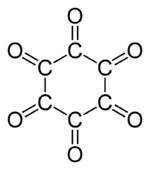
Cyclohexanehexone, also known as hexaketocyclohexane and triquinoyl, is an organic compound with formula C
6O
6, the sixfold ketone of cyclohexane. It is an oxide of carbon (an oxocarbon), a hexamer of carbon monoxide.
The compound is expected to be highly unstable, even less stable than the cyclohexanehexathione analog, and as of 1999 had only been observed as an ionized fragment during mass spectrometry studies.[1][2]
Related compounds
Cyclohexanehexone can be viewed as the neutral counterpart of the rhodizonate anion C
6O2−
6. The singly charged anion C
6O−
6 has been detected in mass spectrometry experiments, formed by oligomerization of carbon monoxide through the formation of molybdenum carbonyls.[3]
According to X-ray diffraction analysis, the reagent traded under the name "cyclohexanehexone octahydrate" or equivalent names is actually dodecahydroxycyclohexane dihydrate—the geminal diol derivative of the six ketone groups with an additional two molecules of water—a solid that decomposes at 95 °C.[4][5]
In 1966, Howard E. Worne of Natick Chemical Industries patented compounds with formulas C
10O
8 and C
14O
10, which can be described as the fusion of two or three molecules of C
6O
6, claimed to be produced by the action of ultraviolet radiation on a hot water solution of the parent compound.[6]
Triquinoyl therapy
In the late 1940s, William J. Hale claimed that "triquinoyl", being a trimer of William Frederick Koch's glyoxylide, should be just as effective as the latter against "diabetes, arthritis, poliomyelitis, and even cancer".[7] Even though there is no research supporting this claim (and Koch's glyoxylide preparations were found to be just distilled water),[8] triquinoyl is still listed as an ingredient of some alternative medicine remedies.[9]
See also
- Cyclopentanepentone
- Ethylenetetracarboxylic dianhydride, an isomer of C
6O
6. - Cyclohexanehexathione, with same structure but with sulfur instead of oxygen.
References
- ↑ Gunther Seitz; Peter Imming (1992). "Oxocarbons and pseudooxocarbons". Chemical Reviews 92 (6): 1227–1260. doi:10.1021/cr00014a004.
- ↑ Schröder, Detlef; Schwarz, Helmut; Dua, Suresh; Blanksby, Stephen J.; Bowie, John H. (1999). "Mass spectrometric studies of the oxocarbons CnOn (n = 3–6)". International Journal of Mass Spectrometry 188 (1–2): 17–25. doi:10.1016/S1387-3806(98)14208-2. ISSN 1387-3806. Bibcode: 1999IJMSp.188...17S.
- ↑ Wyrwas, Richard B.; Jarrold, Caroline Chick (2006). "Production of C6O6– from Oligomerization of CO on Molybdenum Anions". Journal of the American Chemical Society 128 (42): 13688–13689. doi:10.1021/ja0643927. PMID 17044687.
- ↑ "Dodecahydroxycyclohexane dihydrate". Acta Crystallographica E 61 (5): o1393–o1395. 2005. doi:10.1107/S1600536805010007. Bibcode: 2005AcCrE..61O1393K.
- ↑ Person, Willis B.; Williams, Dale G. (1957). "Infrared Spectra and the Structure of Leuconic Acid and Triquinoyl". The Journal of Physical Chemistry 61 (7): 1017–1018. doi:10.1021/j150553a047. ISSN 0022-3654.
- ↑ Worne, Howard E., "Polycarbonyls", US patent 3227641, issued 1996-01-04, assigned to Natick Chemical Industries
- ↑ Hale, William J. (1949). "Farmer Victorious—Money, Mart, and Mother Earth" (reprint). https://williamfkoch.com/pages/farmers/?pID=5&pID2=74.[yes|permanent dead link|dead link}}]
- ↑ Goodrich, William W. (October 15–16, 1986). "FDA Oral History Interview, Goodrich" (PDF) (Interview). Interviewed by Ronald T. Ottes and Fred L. Lofsvold. p. 31.
- ↑ "Import Alert #66-46 – Unapproved Version of Rodaquin". U. S. Food and Drug Administration. 1989. https://www.accessdata.fda.gov/cms_ia/importalert_194.html.
 |