Chemistry:Enol ether
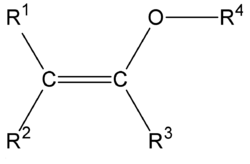

In organic chemistry an enol ether is an alkene with an alkoxy substituent.[1] The general structure is R2C=CR-OR where R = H, alkyl or aryl. A common subfamily of enol ethers are vinyl ethers, with the formula ROCH=CH2. Important enol ethers include the reagent 3,4-dihydropyran and the monomers methyl vinyl ether and ethyl vinyl ether.
Reactions and uses
Akin to enamines, enol ethers are electron-rich alkenes by virtue of the electron-donation from the heteroatom via pi-bonding. Enol ethers have oxonium ion character. By virtue of their bonding situation, enol ethers display distinctive reactivity. In comparison with simple alkenes, enol ethers exhibit enhanced susceptibility to attack by electrophiles such as Bronsted acids. Similarly, they undergo inverse demand Diels-Alder reactions.[2]
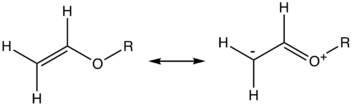
The reactivity of enol ethers is highly dependent on the presence of substituents alpha to oxygen. The vinyl ethers are susceptible to polymerization to give polyvinyl ethers.[3] They also react readily with thiols in the thiol-ene reaction to form thioethers. This makes enol ether-functionalized monomers ideal for polymerization with thiol-based monomers to form thiol-ene networks.[4]
Some vinyl ethers find some use as inhalation anesthetics. Enol ethers bearing α substituents do not polymerize readily. They are mainly of academic interest, e.g. as intermediates in the synthesis of more complex molecules.
The acid-catalyzed addition of hydrogen peroxide to vinyl ethers gives the hydroperoxide:[5]
- C2H5OCH=CH2 + H2O2 → C2H5OCH(OOH)CH3
Nazi Germany used vinyl ether mixtures as rocket propellants during WWII, because their hypergolic combustion with a mixture of nitric and sulfuric acids is relatively insensitive to temperature.[6]: 16
Preparation
Vinyl ethers can be prepared from alcohols by iridium-catalyzed transesterification of vinyl esters, especially the widely available vinyl acetate:[7]
- ROH + CH2=CHOAc → ROCH=CH2 + HOAc
Vinyl ethers can be prepared by reaction of acetylene and alcohols in presence of a base.[8]
Although enol ethers can be considered the ether of the corresponding enolates, they are not prepared by alkylation of enolates. Some enol ethers are prepared from saturated ethers by elimination reactions.[9]
Occurrence in nature
A prominent enol ether is phosphoenol pyruvate.[10]
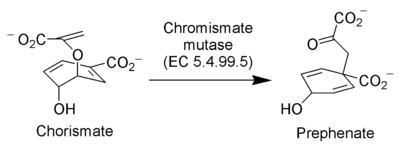
The enzyme chorismate mutase catalyzes the Claisen rearrangement of the enol ether called chorismate to prephenate, an intermediate in the biosynthesis of phenylalanine and tyrosine.[11]
Batyl alcohol and related glycyl ethers are susceptible to dehydrogenation catalyzed unsaturases to give the vinyl ethers called plasmalogens:[12]
- HOCH
2CH(OH)CH
2OC
18H
37 + [O] → HOCH
2CH(OH)CH
2OCH=CHC
16H
35 + H
2O

See also
References
- ↑ Jonathan Clayden; Greeves, Nick; Stuart Warren (2012). Organic Chemistry (2nd ed.). Oxford University Press. p. 295. ISBN 978-0-19-927029-3.
- ↑ Percy S. Manchand (2001). "Ethyl Vinyl Ether". EEROS. doi:10.1002/047084289X.re125. ISBN 0-471-93623-5.
- ↑ Gerd Schröder (2012). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_011.
- ↑ Hoyle, Charles E.; Bowman, Christopher N. (2010). "Thiol–Ene Click Chemistry". Angewandte Chemie International Edition 49 (9): 1540–1573. doi:10.1002/anie.200903924.
- ↑ Milas, Nicholas A.; Peeler, Robert L.; Mageli, Orville L. (1954). "Organic Peroxides. XIX. α-Hydroperoxyethers and Related Peroxides". Journal of the American Chemical Society 76 (9): 2322–2325. doi:10.1021/ja01638a012.
- ↑ Clark, John Drury (23 May 2018). Ignition!: An Informal History of Liquid Rocket Propellants. Rutgers University Press. pp. 302. ISBN 978-0-8135-9918-2. OCLC 281664. https://books.google.com/books?id=BdU4DwAAQBAJ&q=vinyl%20ether.
- ↑ Tomotaka Hirabayashi; Satoshi Sakaguchi; Yasutaka Ishii (2005). "Iridium-catalyzed Synthesis of Vinyl Ethers from Alcohols and Vinyl Acetate". Org. Synth. 82: 55. doi:10.15227/orgsyn.082.0055.
- ↑ Ernst Hofmann; Hans‐Joachim Klimisch; René Backes; Regina Vogelsang; Lothar Franz; Robert Feuerhake (2011). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_435.pub2.
- ↑ Carl Kaiser; Joseph Weinstock (1976). "Alkenes Via Hofmann Elimination: Use of Ion-exchange Resin for Preparation of Quaternary Ammonium Hydroxides: Diphenylmethyl Vinyl Ether". Org. Synth. 55: 3. doi:10.15227/orgsyn.055.0003.
- ↑ Walsh, C.T.; Benson, T.E.; Kim, D.H.; Lees, W.J. (1996). "The versatility of phosphoenolpyruvate and its vinyl ether products in biosynthesis". Chemistry & Biology 3 (2): 83–91. doi:10.1016/s1074-5521(96)90282-3. PMID 8807832.
- ↑ Ganem, B. (1996). "The Mechanism of the Claisen Rearrangement: Déjà Vu All over Again". Angew. Chem. Int. Ed. Engl. 35 (9): 936–945. doi:10.1002/anie.199609361.
- ↑ Taguchi, Hiroyasu; Armarego, Wilfred L. F. (1998). "Glyceryl-Ether Monooxygenase [EC 1.14.16.5]. A Microsomal Enzyme of Ether Lipid Metabolism". Medicinal Research Reviews 18 (1): 43–89. doi:10.1002/(SICI)1098-1128(199801)18:1<43::AID-MED3>3.0.CO;2-S. PMID 9436181.
- ↑ "Structural Requirements of Strigolactones for Shoot Branching Inhibition in Rice and Arabidopsis". Plant & Cell Physiology 56 (6): 1059–72. June 2015. doi:10.1093/pcp/pcv028. PMID 25713176.
 |
