Chemistry:Enzastaurin
From HandWiki
| |
| Names | |
|---|---|
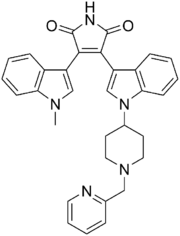
| Preferred IUPAC name
3-(1-Methyl-1H-indol-3-yl)-4-(1-{1-[(pyridin-2-yl)methyl]piperidin-4-yl}-1H-indol-3-yl)-1H-pyrrole-2,5-dione | |
| Other names
LY-317615
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C32H29N5O2 | |
| Molar mass | 515.617 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Enzastaurin is a synthetic bisindolylmaleimide with potential antineoplastic activity. Binding to the ATP-binding site, enzastaurin selectively inhibits protein kinase C beta, an enzyme involved in the induction of vascular endothelial growth factor (VEGF)-stimulated neo-angiogenesis. This agent may decrease tumor blood supply, preventing growth.
Trials
In 2013 it failed a phase III clinical trial for lymphoma.[1]
In 2022, there is an upcoming initial trial called PREVEnt to look into the effectiveness of Enzastaurin for the treatment of Vascular Elhers-Danlos syndrome (vEDS). [2][3][4]
References
- ↑ Lilly Halts Development of Lymphoma Drug After Phase III Failure
- ↑ "New vEDS clinical trial" (in en-US). https://preventvedstrial.com/.
- ↑ "Aytu BioPharma Adds Late-Stage Pediatric Onset Rare Disease Asset to Development Pipeline from Rumpus Therapeutics" (in en-US). https://www.biospace.com/article/aytu-biopharma-adds-late-stage-pediatric-onset-rare-disease-asset-to-development-pipeline-from-rumpus-therapeutics/.
- ↑ "Clinical Trials" (in en). https://www.fightveds.org/clinical-trials.
External links
- Enzastaurin hydrochloride, National Institutes of Health
 |

