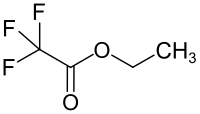
Chemistry:Ethyl trifluoroacetate
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C4H5F3O2 | |
| Molar mass | 142.077 g·mol−1 |
| Density | 1.1952 g/cm3 (16.7 °C) |
| Boiling point | 61 °C (142 °F; 334 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ethyl trifluoroacetate is a chemical compound from the trifluoroacetate group.
Production
Ethyl trifluoroacetate can be obtained by reacting 2,4,6-tris-(trifluoromethyl)-1,3,5-triazine with ethanol in the presence of hydrochloric acid. The former, in turn, can be prepared by a two-step reaction starting from trichloroacetonitrile by reaction with hydrogen chloride and fluorination of the intermediate with antimony trifluoride.[1]
The compound can also be obtained by reacting trifluoroacetic acid or sodium trifluoroacetate[2] with ethanol.[3]
Properties
Ethyl trifluoroacetate is a colorless and odorless liquid that is sparingly soluble in water but miscible with chloroform and methanol. The compound exists in the gas phase in two more conformal[clarification needed] forms.[4]
Use
Ethyl trifluoroacetate is used as an intermediate in organic synthesis to prepare organic fluorine compounds such as 3-ethyl-1-methylimidazolium trifluoroacetate (EMITA). It is also used in the synthesis of various pharmaceutically active molecules and agricultural products, and is also useful for the preparation of trifluoroacetylated compounds. The trifluoroacetyl group is widely used as an amine protecting group in organic synthesis because it can be easily removed under mild conditions.[5]
References
- ↑ T. R. Norton (1950). "A New Synthesis of Ethyl Trifluoroacetate". Journal of the American Chemical Society 72 (8): 3527–3528. doi:10.1021/ja01164a056. ISSN 0002-7863.
- ↑ Murray, R. L.; Babcock, J. H. (1946). The Preparation of Sodium Trifluoroacetate and Ethyl Trifluoroacetate. Atomic Energy Commission. https://books.google.com/books?id=4pnMwpxThpUC.
- ↑ Google Patents: US4879407A - Process for the preparation of ethyl trifluoroacetate - Google Patents, retrieved 7 August 2022
- ↑ "Experimental and theoretical structure and vibrational analysis of ethyl trifluoroacetate, CF3CO2CH2CH3: Structure and vibrational analysis of CF3CO2CH2CH3". Journal of Raman Spectroscopy 41 (10): 1357–1368. 2010. doi:10.1002/jrs.2550.
- ↑ Xu, Daqiang; Prasad, Kapa; Repic, Oljan; Blacklock, Thomas J. (1995). "Ethyl trifluoroacetate: a powerful reagent for differentiating amino groups" (in German). Tetrahedron Letters 36 (41): 7357–7360. doi:10.1016/0040-4039(95)01655-4. ISSN 0040-4039.
 |

