Chemistry:Eucaine
 | |
| Clinical data | |
|---|---|
| Trade names | Beta-Eucaine |
| Other names |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
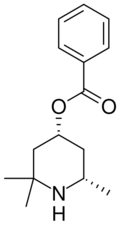
| Formula | C15H21NO2 |
| Molar mass | 247.338 g·mol−1 |
| 3D model (JSmol) | |
| |
Eucaine, also known as β-eucaine or Betacaine, is a drug that was previously used as a local anesthetic.[1] It was designed as an analog of cocaine and was one of the first synthetic chemical compounds to find general use as an anesthetic.[2] It is a white, crystalline solid. Prior to World War I, Britain imported eucaine from Germany.[3] During the war, a team including Jocelyn Field Thorpe and Martha Annie Whiteley developed a synthesis in Britain.[3]
The brand name Betacaine can sometimes refer to a preparation containing lidocaine, not eucaine.
Synthesis
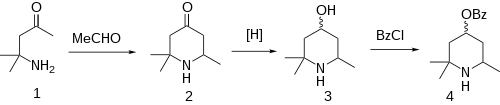
Condensation of diacetonamine [625-04-7] (1) with acetaldehyde (paraldehyde) rather than acetone gives the piperidone containing one less methyl group, i.e. 2,2,6-trimethylpiperidin-4-one [3311-23-7] (2). Reduction of the ketone with sodium amalgam gives the alcohol as a mixture of isomers, 2,2,6-trimethylpiperidin-4-ol (3). Benzoylation then affords beta-eucaine (4).
See also
- α-Eucaine, a related local anesthetic
References
- ↑ Drug Discovery: A History. John Wiley & Sons. 31 October 2005. pp. 127–9. ISBN 978-0-470-01552-0. https://books.google.com/books?id=jglFsz5EJR8C&pg=PA127.
- ↑ The Alkaloids: Chemistry and Physiology. Elsevier. 12 May 2014. pp. 213–4. ISBN 978-1-4832-2192-2. https://books.google.com/books?id=DvbJCgAAQBAJ&pg=PA213.
- ↑ 3.0 3.1 "Martha Annie Whiteley (1866-1956): Chemist and Editor". Bulletin for the History of Chemistry 8: 42–45. 1997. http://www.scs.illinois.edu/~mainzv/HIST/bulletin_open_access/num20/num20%20p42-45.pdf.
- ↑ Organic medical chemicals, by M. Barrowliff, 98-99, 1921.
- ↑ Harries, C. (1918). "Untersuchungen über die cyclischen Acetonbasen". Justus Liebig's Annalen der Chemie 417 (2-3): 107–191. doi:10.1002/jlac.19184170202.
- ↑ King, Harold (1924). "VII.—Stereoisomerism and local anœsthetic action in the β-eucaine group. Resolution of β- and iso-β-eucaine". J. Chem. Soc., Trans. 125 (0): 41–57. doi:10.1039/CT9242500041.
External links
 |

