Biology:Cocaine
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | kə(ʊ)ˈkeɪn |
| Trade names | Neurocaine,[1] Goprelto,[2] Numbrino,[3] others |
| Other names | Coke, blow, snow, crack (in free base form) |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| License data | |
| Pregnancy category |
|
| Dependence liability | Physical: None Psychological: High[5] |
| Addiction liability | High[6] |
| Routes of administration | Topical, by mouth, insufflation, intravenous, inhalation |
| Drug class |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability |
|
| Metabolism | Liver, CYP3A4 |
| Metabolites | Norcocaine, benzoylecgonine, cocaethylene |
| Onset of action | Seconds to minutes[13] |
| Duration of action | 20 to 90 minutes[13] |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
| Formula | C17H21NO4 |
| Molar mass | 303.358 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 98 °C (208 °F) |
| Boiling point | 187 °C (369 °F) |
| Solubility in water | 1.8g/L (22 °C) |
| |
| |
| | |
Cocaine (from French: cocaïne, from Spanish: coca, ultimately from Quechua: kúka)[14] is a tropane alkaloid that acts as a central nervous system (CNS) stimulant. As an extract, it is mainly used recreationally, and often illegally for its euphoric and rewarding effects. It is also used in medicine by Indigenous South Americans for various purposes and rarely, but more formally, as a local anaesthetic or diagnostic tool by medical practitioners in more developed countries. It is primarily obtained from the leaves of two Coca species native to South America: Erythroxylum coca and E. novogranatense.[15][16] After extraction from the plant, and further processing into cocaine hydrochloride (powdered cocaine), the drug is administered by being either snorted, applied topically to the mouth, or dissolved and injected into a vein. It can also then be turned into free base form (typically crack cocaine), in which it can be heated until sublimated and then the vapours can be inhaled.[13]
Cocaine stimulates the reward pathway in the brain.[16] Mental effects may include an intense feeling of happiness, sexual arousal, loss of contact with reality, or agitation.[13] Physical effects may include a fast heart rate, sweating, and dilated pupils.[13] High doses can result in high blood pressure or high body temperature.[17] Onset of effects can begin within seconds to minutes of use, depending on method of delivery, and can last between five and ninety minutes.[13] As cocaine also has numbing and blood vessel constriction properties, it is occasionally used during surgery on the throat or inside of the nose to control pain, bleeding, and vocal cord spasm.[18]
Cocaine crosses the blood–brain barrier via a proton-coupled organic cation antiporter[19][20] and (to a lesser extent) via passive diffusion across cell membranes.[21] Cocaine blocks the dopamine transporter,[22] inhibiting reuptake of dopamine from the synaptic cleft into the pre-synaptic axon terminal; the higher dopamine levels in the synaptic cleft increase dopamine receptor activation in the post-synaptic neuron,[23][24] causing euphoria and arousal.[25] Cocaine also blocks the serotonin transporter and norepinephrine transporter, inhibiting reuptake of serotonin and norepinephrine from the synaptic cleft into the pre-synaptic axon terminal and increasing activation of serotonin receptors and norepinephrine receptors in the post-synaptic neuron, contributing to the mental and physical effects of cocaine exposure.[7]
A single dose of cocaine induces tolerance to the drug's effects.[26] Repeated use is likely to result in addiction. Addicts who abstain from cocaine may experience craving and drug withdrawal symptoms, with depression, decreased libido, decreased ability to feel pleasure, and fatigue being most common.[16] Use of cocaine increases the overall risk of death, and intravenous use potentially increases the risk of trauma and infectious diseases such as blood infections and HIV through the use of shared paraphernalia. It also increases risk of stroke, heart attack, cardiac arrhythmia, lung injury (when smoked), and sudden cardiac death.[16][27] Illicitly sold cocaine can be adulterated with fentanyl, local anesthetics, levamisole, cornstarch, quinine, or sugar, which can result in additional toxicity.[28][29] In 2017, the Global Burden of Disease study found that cocaine use caused around 7,300 deaths annually.[30]
Uses
Coca leaves have been used by Andean civilizations since ancient times.[28] In ancient Wari culture,[31] Inca culture, and through modern successor indigenous cultures of the Andes mountains, coca leaves are chewed, taken orally in the form of a tea, or alternatively, prepared in a sachet wrapped around alkaline burnt ashes, and held in the mouth against the inner cheek; it has traditionally been used to combat the effects of cold, hunger, and altitude sickness.[32][33] Cocaine was first isolated from the leaves in 1860.[16]
Globally, in 2019, cocaine was used by an estimated 20 million people (0.4% of adults aged 15 to 64 years). The highest prevalence of cocaine use was in Australia and New Zealand (2.1%), followed by North America (2.1%), Western and Central Europe (1.4%), and South and Central America (1.0%).[34] Since 1961, the Single Convention on Narcotic Drugs has required countries to make recreational use of cocaine a crime.[35] In the United States, cocaine is regulated as a Schedule II drug under the Controlled Substances Act, meaning that it has a high potential for abuse but has an accepted medical use.[36] While rarely used medically today, its accepted uses are as a topical local anesthetic for the upper respiratory tract as well as to reduce bleeding in the mouth, throat and nasal cavities.[citation needed]
Medical
Cocaine eye drops are frequently used by neurologists when examining patients suspected of having Horner syndrome. In Horner syndrome, sympathetic innervation to the eye is blocked. In a healthy eye, cocaine will stimulate the sympathetic nerves by inhibiting norepinephrine reuptake, and the pupil will dilate; if the patient has Horner syndrome, the sympathetic nerves are blocked, and the affected eye will remain constricted or dilate to a lesser extent than the opposing (unaffected) eye which also receives the eye drop test. If both eyes dilate equally, the patient does not have Horner syndrome.[37]

Topical cocaine is sometimes used as a local numbing agent and vasoconstrictor to help control pain and bleeding with surgery of the nose, mouth, throat or lacrimal duct. Although some absorption and systemic effects may occur, the use of cocaine as a topical anesthetic and vasoconstrictor is generally safe, rarely causing cardiovascular toxicity, glaucoma, and pupil dilation.[38][39] Occasionally, cocaine is mixed with adrenaline and sodium bicarbonate and used topically for surgery, a formulation called Moffett's solution.[40]
Cocaine hydrochloride (Goprelto), an ester local anesthetic, was approved for medical use in the United States in December 2017, and is indicated for the introduction of local anesthesia of the mucous membranes for diagnostic procedures and surgeries on or through the nasal cavities of adults.[41][2] Cocaine hydrochloride (Numbrino) was approved for medical use in the United States in January 2020.[42][3]
The most common adverse reactions in people treated with Goprelto are headache and epistaxis.[2] The most common adverse reactions in people treated with Numbrino are hypertension, tachycardia, and sinus tachycardia.[3]
Recreational
Cocaine is a central nervous system stimulant.[43] Its effects can last from 15 minutes to an hour. The duration of cocaine's effects depends on the amount taken and the route of administration.[44] Cocaine can be in the form of fine white powder and has a bitter taste. Crack cocaine is a smokeable form of cocaine made into small "rocks" by processing cocaine with sodium bicarbonate (baking soda) and water.[13][45] Crack cocaine is referred to as "crack" because of the crackling sounds it makes when heated.[13]
Cocaine use leads to increases in alertness, feelings of well-being and euphoria, increased energy and motor activity, and increased feelings of competence and sexuality.[46]
Analysis of the correlation between the use of 18 various psychoactive substances shows that cocaine use correlates with other "party drugs" (such as ecstasy or amphetamines), as well as with heroin and benzodiazepines use, and can be considered as a bridge between the use of different groups of drugs.[47]
Coca leaves
It is legal for people to use coca leaves in some Andean nations, such as Peru and Bolivia, where they are chewed, consumed in the form of tea, or are sometimes incorporated into food products.[48] Coca leaves are typically mixed with an alkaline substance (such as lime) and chewed into a wad that is retained in the buccal pouch (mouth between gum and cheek, much the same as chewing tobacco is chewed) and sucked of its juices. The juices are absorbed slowly by the mucous membrane of the inner cheek and by the gastrointestinal tract when swallowed. Alternatively, coca leaves can be infused in liquid and consumed like tea. Coca tea, an infusion of coca leaves, is also a traditional method of consumption. The tea has often been recommended for travelers in the Andes to prevent altitude sickness.[49] Its actual effectiveness has never been systematically studied.[49]
In 1986 an article in the Journal of the American Medical Association revealed that U.S. health food stores were selling dried coca leaves to be prepared as an infusion as "Health Inca Tea". While the packaging claimed it had been "decocainized", no such process had actually taken place. The article stated that drinking two cups of the tea per day gave a mild stimulation, increased heart rate, and mood elevation, and the tea was essentially harmless.[50]
Insufflation

Nasal insufflation (known colloquially as "snorting", "sniffing", or "blowing") is a common method of ingestion of recreational powdered cocaine.[51] The drug coats and is absorbed through the mucous membranes lining the nasal passages. Cocaine's desired euphoric effects are delayed when snorted through the nose by about five minutes. This occurs because cocaine's absorption is slowed by its constricting effect on the blood vessels of the nose.[13] Insufflation of cocaine also leads to the longest duration of its effects (60–90 minutes).[13] When insufflating cocaine, absorption through the nasal membranes is approximately 30–60%[52]
In a study of cocaine users, the average time taken to reach peak subjective effects was 14.6 minutes.[53] Any damage to the inside of the nose is due to cocaine constricting blood vessels — and therefore restricting blood and oxygen/nutrient flow — to that area.
Rolled up banknotes, hollowed-out pens, cut straws, pointed ends of keys, specialized spoons,[54] long fingernails, and (clean) tampon applicators are often used to insufflate cocaine. The cocaine typically is poured onto a flat, hard surface (such as a mobile phone screen, mirror, CD case or book) and divided into "bumps", "lines" or "rails", and then insufflated.[55] A 2001 study reported that the sharing of straws used to "snort" cocaine can spread blood diseases such as hepatitis C.[56]
Injection
Subjective effects not commonly shared with other methods of administration include a ringing in the ears moments after injection (usually when over 120 milligrams) lasting two to 5 minutes including tinnitus and audio distortion. This is colloquially referred to as a "bell ringer". In a study of cocaine users, the average time taken to reach peak subjective effects was 3.1 minutes.[53] The euphoria passes quickly. Aside from the toxic effects of cocaine, there is also the danger of circulatory emboli from the insoluble substances that may be used to cut the drug. As with all injected illicit substances, there is a risk of the user contracting blood-borne infections if sterile injecting equipment is not available or used.
An injected mixture of cocaine and heroin, known as "speedball", is a particularly dangerous combination, as the converse effects of the drugs actually complement each other, but may also mask the symptoms of an overdose. It has been responsible for numerous deaths, including celebrities such as comedians/actors John Belushi and Chris Farley, Mitch Hedberg, River Phoenix, grunge singer Layne Staley and actor Philip Seymour Hoffman. Experimentally, cocaine injections can be delivered to animals such as fruit flies to study the mechanisms of cocaine addiction.[57]
Inhalation
The onset of cocaine's euphoric effects is fastest with inhalation, beginning after 3–5 seconds.[13] However, inhalation gives the shortest duration of euphoria (5–15 minutes).[13] Cocaine is smoked by inhaling the vapor produced when free base cocaine is heated to the point of sublimation.[58] In a 2000 Brookhaven National Laboratory medical department study, based on self-reports of 32 people who used cocaine who participated in the study, "peak high" was found at a mean of 1.4 ± 0.5 minutes.[53] Pyrolysis products of cocaine that occur only when heated/smoked have been shown to change the effect profile, i.e. anhydroecgonine methyl ester, when co-administered with cocaine, increases the dopamine in CPu and NAc brain regions, and has M1 — and M3 — receptor affinity.[59]
Smoking freebase cocaine is often accomplished using a pipe made from a small glass tube, often taken from "love roses", small glass tubes with a paper rose that are promoted as romantic gifts.[60] These are sometimes called "stems", "horns", "blasters" and "straight shooters". A small piece of clean heavy copper or occasionally stainless steel scouring pad – often called a "brillo" (actual Brillo Pads contain soap, and are not used) or "chore" (named for Chore Boy brand copper scouring pads) – serves as a reduction base and flow modulator in which the "rock" can be melted and boiled to vapor. Crack is smoked by placing it at the end of the pipe; a flame held close to it produces vapor, which is then inhaled by the smoker. The effects felt almost immediately after smoking, are very intense and do not last long — usually 2 to 10 minutes.[61] When smoked, cocaine is sometimes combined with other drugs, such as cannabis, often rolled into a joint or blunt.
Effects
-
Opioid involvement in cocaine overdose deaths in the US. The pale green line is cocaine without any opioid (bottom line in 2017). The yellow line is cocaine and synthetic opioids other than methadone (top line in 2017).[63]
-
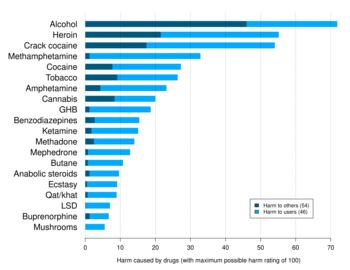
Delphic analysis regarding 20 popular recreational drugs based on expert opinion in the UK. Cocaine was ranked the 2nd in dependence and physical harm and 3rd in social harm.[64]
Acute
Acute exposure to cocaine has many effects on humans, including euphoria, increases in heart rate and blood pressure, and increases in cortisol secretion from the adrenal gland.[65] In humans with acute exposure followed by continuous exposure to cocaine at a constant blood concentration, the acute tolerance to the chronotropic cardiac effects of cocaine begins after about 10 minutes, while acute tolerance to the euphoric effects of cocaine begins after about one hour.[26][66][67][68] With excessive or prolonged use, the drug can cause itching, fast heart rate, and paranoid delusions or sensations of insects crawling on the skin.[69] Intranasal cocaine and crack use are both associated with pharmacological violence. Aggressive behavior may be displayed by both addicts and casual users. Cocaine can induce psychosis characterized by paranoia, impaired reality testing, hallucinations, irritability, and physical aggression. Cocaine intoxication can cause hyperawareness, hypervigilance, and psychomotor agitation and delirium. Consumption of large doses of cocaine can cause violent outbursts, especially by those with preexisting psychosis. Crack-related violence is also systemic, relating to disputes between crack dealers and users.[70] Acute exposure may induce cardiac arrhythmias, including atrial fibrillation, supraventricular tachycardia, ventricular tachycardia, and ventricular fibrillation. Acute exposure may also lead to angina, heart attack, and congestive heart failure.[71] Cocaine overdose may cause seizures, abnormally high body temperature and a marked elevation of blood pressure, which can be life-threatening,[69] abnormal heart rhythms,[72] and death.[72] Anxiety, paranoia, and restlessness can also occur, especially during the comedown. With excessive dosage, tremors, convulsions and increased body temperature are observed.[43] Severe cardiac adverse events, particularly sudden cardiac death, become a serious risk at high doses due to cocaine's blocking effect on cardiac sodium channels.[72] Incidental exposure of the eye to sublimated cocaine while smoking crack cocaine can cause serious injury to the cornea and long-term loss of visual acuity.[73]
Chronic
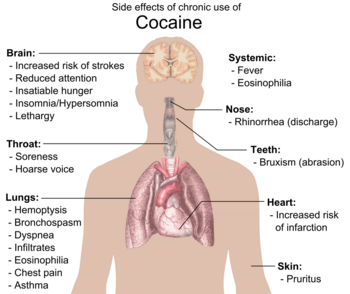
Although it has been commonly asserted, the available evidence does not show that chronic use of cocaine is associated with broad cognitive deficits.[74] Research is inconclusive on age-related loss of striatal dopamine transporter (DAT) sites, suggesting cocaine has neuroprotective or neurodegenerative properties for dopamine neurons.[75][76][77] Exposure to cocaine may lead to the breakdown of the blood–brain barrier.[78][79]
Physical side effects from chronic smoking of cocaine include coughing up blood, bronchospasm, itching, fever, diffuse alveolar infiltrates without effusions, pulmonary and systemic eosinophilia, chest pain, lung trauma, sore throat, asthma, hoarse voice, dyspnea (shortness of breath), and an aching, flu-like syndrome. Cocaine constricts blood vessels, dilates pupils, and increases body temperature, heart rate, and blood pressure. It can also cause headaches and gastrointestinal complications such as abdominal pain and nausea. A common but untrue belief is that the smoking of cocaine chemically breaks down tooth enamel and causes tooth decay. Cocaine can cause involuntary tooth grinding, known as bruxism, which can deteriorate tooth enamel and lead to gingivitis.[80] Additionally, stimulants like cocaine, methamphetamine, and even caffeine cause dehydration and dry mouth. Since saliva is an important mechanism in maintaining one's oral pH level, people who use cocaine over a long period of time who do not hydrate sufficiently may experience demineralization of their teeth due to the pH of the tooth surface dropping too low (below 5.5). Cocaine use also promotes the formation of blood clots.[13] This increase in blood clot formation is attributed to cocaine-associated increases in the activity of plasminogen activator inhibitor, and an increase in the number, activation, and aggregation of platelets.[13]
Chronic intranasal usage can degrade the cartilage separating the nostrils (the septum nasi), leading eventually to its complete disappearance. Due to the absorption of the cocaine from cocaine hydrochloride, the remaining hydrochloride forms a dilute hydrochloric acid.[81]
Illicitly-sold cocaine may be contaminated with levamisole.[82] Levamisole may accentuate cocaine's effects.[83] Levamisole-adulterated cocaine has been associated with autoimmune disease.[84]
Cocaine use leads to an increased risk of hemorrhagic and ischemic strokes.[45] Cocaine use also increases the risk of having a heart attack.[85]
Addiction
Relatives of persons with cocaine addiction have an increased risk of cocaine addiction.[86] Cocaine addiction occurs through ΔFosB overexpression in the nucleus accumbens, which results in altered transcriptional regulation in neurons within the nucleus accumbens. ΔFosB levels have been found to increase upon the use of cocaine.[87] Each subsequent dose of cocaine continues to increase ΔFosB levels with no ceiling of tolerance. Elevated levels of ΔFosB leads to increases in brain-derived neurotrophic factor (BDNF) levels, which in turn increases the number of dendritic branches and spines present on neurons involved with the nucleus accumbens and prefrontal cortex areas of the brain. This change can be identified rather quickly, and may be sustained weeks after the last dose of the drug.
Transgenic mice exhibiting inducible expression of ΔFosB primarily in the nucleus accumbens and dorsal striatum exhibit sensitized behavioural responses to cocaine.[88] They self-administer cocaine at lower doses than control,[89] but have a greater likelihood of relapse when the drug is withheld.[89][90] ΔFosB increases the expression of AMPA receptor subunit GluR2[88] and also decreases expression of dynorphin, thereby enhancing sensitivity to reward.[90]
DNA damage is increased in the brain of rodents by administration of cocaine.[91][92] During DNA repair of such damages, persistent chromatin alterations may occur such as methylation of DNA or the acetylation or methylation of histones at the sites of repair.[93] These alterations can be epigenetic scars in the chromatin that contribute to the persistent epigenetic changes found in cocaine addiction.
In humans, cocaine abuse may cause structural changes in brain connectivity, though it is unclear to what extent these changes are permanent.[94]
Dependence and withdrawal
Cocaine dependence develops after even brief periods of regular cocaine use[95] and produces a withdrawal state with emotional-motivational deficits upon cessation of cocaine use.
During pregnancy
Crack baby is a term for a child born to a mother who used crack cocaine during her pregnancy. The threat that cocaine use during pregnancy poses to the fetus is now considered exaggerated.[96] Studies show that prenatal cocaine exposure (independent of other effects such as, for example, alcohol, tobacco, or physical environment) has no appreciable effect on childhood growth and development.[97] However, the official opinion of the National Institute on Drug Abuse of the United States warns about health risks while cautioning against stereotyping:
Many recall that "crack babies", or babies born to mothers who used crack cocaine while pregnant, were at one time written off by many as a lost generation. They were predicted to suffer from severe, irreversible damage, including reduced intelligence and social skills. It was later found that this was a gross exaggeration. However, the fact that most of these children appear normal should not be over-interpreted as indicating that there is no cause for concern. Using sophisticated technologies, scientists are now finding that exposure to cocaine during fetal development may lead to subtle, yet significant, later deficits in some children, including deficits in some aspects of cognitive performance, information-processing, and attention to tasks—abilities that are important for success in school.[98]
There are also warnings about the threat of breastfeeding: The March of Dimes said "it is likely that cocaine will reach the baby through breast milk," and advises the following regarding cocaine use during pregnancy:
Cocaine use during pregnancy can affect a pregnant woman and her unborn baby in many ways. During the early months of pregnancy, it may increase the risk of miscarriage. Later in pregnancy, it can trigger preterm labor (labor that occurs before 37 weeks of pregnancy) or cause the baby to grow poorly. As a result, cocaine-exposed babies are more likely than unexposed babies to be born with low birth weight (less than 5.5 lb or 2.5 kg). Low-birthweight babies are 20 times more likely to die in their first month of life than normal-weight babies, and face an increased risk of lifelong disabilities such as mental retardation and cerebral palsy. Cocaine-exposed babies also tend to have smaller heads, which generally reflect smaller brains. Some studies suggest that cocaine-exposed babies are at increased risk of birth defects, including urinary tract defects and, possibly, heart defects. Cocaine also may cause an unborn baby to have a stroke, irreversible brain damage, or a heart attack.[99]
Mortality
Persons with regular or problematic use of cocaine have a significantly higher rate of death, and are specifically at higher risk of traumatic deaths and deaths attributable to infectious disease.[100]
Pharmacology
Pharmacokinetics
The extent of absorption of cocaine into the systemic circulation after nasal insufflation is similar to that after oral ingestion. The rate of absorption after nasal insufflation is limited by cocaine-induced vasoconstriction of capillaries in the nasal mucosa. Onset of absorption after oral ingestion is delayed because cocaine is a weak base with a pKa of 8.6, and is thus in an ionized form that is poorly absorbed from the acidic stomach and easily absorbed from the alkaline duodenum.[12] The rate and extent of absorption from inhalation of cocaine is similar or greater than with intravenous injection, as inhalation provides access directly to the pulmonary capillary bed. The delay in absorption after oral ingestion may account for the popular belief that cocaine bioavailability from the stomach is lower than after insufflation. Compared with ingestion, the faster absorption of insufflated cocaine results in quicker attainment of maximum drug effects. Snorting cocaine produces maximum physiological effects within 40 minutes and maximum psychotropic effects within 20 minutes. Physiological and psychotropic effects from nasally insufflated cocaine are sustained for approximately 40–60 minutes after the peak effects are attained.[101]
Cocaine crosses the blood–brain barrier via both a proton-coupled organic cation antiporter[19][20] and (to a lesser extent) via passive diffusion across cell membranes.[21] As of September 2022, the gene or genes encoding the human proton-organic cation antiporter had not been identified.[102]
Cocaine has a short elimination half life of 0.7–1.5 hours and is extensively metabolized by plasma esterases and also by liver cholinesterases, with only about 1% excreted unchanged in the urine.[13] The metabolism is dominated by hydrolytic ester cleavage, so the eliminated metabolites consist mostly of benzoylecgonine (BE), the major metabolite, and other metabolites in lesser amounts such as ecgonine methyl ester (EME) and ecgonine.[103][13] Further minor metabolites of cocaine include norcocaine, p-hydroxycocaine, m-hydroxycocaine, p-hydroxybenzoylecgonine (pOHBE), and m-hydroxybenzoylecgonine.[104] If consumed with alcohol, cocaine combines with alcohol in the liver to form cocaethylene.[13] Studies have suggested cocaethylene is more euphoric, and has a higher cardiovascular toxicity than cocaine by itself.[13]
Depending on liver and kidney function, cocaine metabolites are detectable in urine. Benzoylecgonine can be detected in urine within four hours after cocaine intake and remains detectable in concentrations greater than 150 ng/mL typically for up to eight days after cocaine is used. Detection of cocaine metabolites in hair is possible in regular users until after the sections of hair grown during the period of cocaine use are cut or fall out.[105]
Pharmacodynamics
The pharmacodynamics of cocaine involve the complex relationships of neurotransmitters (inhibiting monoamine uptake in rats with ratios of about: serotonin:dopamine = 2:3, serotonin:norepinephrine = 2:5).[106][16] The most extensively studied effect of cocaine on the central nervous system is the blockade of the dopamine transporter protein. Dopamine neurotransmitter released during neural signaling is normally recycled via the transporter; i.e., the transporter binds the transmitter and pumps it out of the synaptic cleft back into the presynaptic neuron, where it is taken up into storage vesicles. Cocaine binds tightly at the dopamine transporter forming a complex that blocks the transporter's function. The dopamine transporter can no longer perform its reuptake function, and thus dopamine accumulates in the synaptic cleft. The increased concentration of dopamine in the synapse activates post-synaptic dopamine receptors, which makes the drug rewarding and promotes the compulsive use of cocaine.[107]
Cocaine affects certain serotonin (5-HT) receptors; in particular, it has been shown to antagonize the 5-HT3 receptor, which is a ligand-gated ion channel. An overabundance of 5-HT3 receptors is reported in cocaine-conditioned rats, though 5-HT3's role is unclear.[108] The 5-HT2 receptor (particularly the subtypes 5-HT2A, 5-HT2B and 5-HT2C) are involved in the locomotor-activating effects of cocaine.[109]
Cocaine has been demonstrated to bind as to directly stabilize the DAT transporter on the open outward-facing conformation. Further, cocaine binds in such a way as to inhibit a hydrogen bond innate to DAT. Cocaine's binding properties are such that it attaches so this hydrogen bond will not form and is blocked from formation due to the tightly locked orientation of the cocaine molecule. Research studies have suggested that the affinity for the transporter is not what is involved in the habituation of the substance so much as the conformation and binding properties to where and how on the transporter the molecule binds.[110]
Sigma receptors are affected by cocaine, as cocaine functions as a sigma ligand agonist.[111] Further specific receptors it has been demonstrated to function on are NMDA and the D1 dopamine receptor.[112]
Cocaine also blocks sodium channels, thereby interfering with the propagation of action potentials;[113][72] thus, like lignocaine and novocaine, it acts as a local anesthetic. It also functions on the binding sites to the dopamine and serotonin sodium dependent transport area as targets as separate mechanisms from its reuptake of those transporters; unique to its local anesthetic value which makes it in a class of functionality different from both its own derived phenyltropanes analogues which have that removed. In addition to this, cocaine has some target binding to the site of the κ-opioid receptor.[114] Cocaine also causes vasoconstriction, thus reducing bleeding during minor surgical procedures. Recent research points to an important role of circadian mechanisms[115] and clock genes[116] in behavioral actions of cocaine.
Cocaine is known to suppress hunger and appetite by increasing co-localization of sigma σ1R receptors and ghrelin GHS-R1a receptors at the neuronal cell surface, thereby increasing ghrelin-mediated signaling of satiety[117] and possibly via other effects on appetitive hormones.[118] Chronic users may lose their appetite and can experience severe malnutrition and significant weight loss.
Cocaine effects, further, are shown to be potentiated for the user when used in conjunction with new surroundings and stimuli, and otherwise novel environs.[119]
Chemistry
Appearance


Cocaine in its purest form is a white, pearly product. Cocaine appearing in powder form is a salt, typically cocaine hydrochloride. Street cocaine is often adulterated or "cut" with talc, lactose, sucrose, glucose, mannitol, inositol, caffeine, procaine, phencyclidine, phenytoin, lignocaine, strychnine, levamisole, amphetamine, or heroin.[120][dubious ]
Crack cocaine looks like irregular shaped white rocks.[121]
Forms
Salts
Cocaine — a tropane alkaloid — is a weakly alkaline compound, and can therefore combine with acidic compounds to form salts. The hydrochloride (HCl) salt of cocaine is by far the most commonly encountered, although the sulfate (SO42−) and the nitrate (NO3−) salts are occasionally seen. Different salts dissolve to a greater or lesser extent in various solvents — the hydrochloride salt is polar in character and is quite soluble in water.[122]
Base
As the name implies, "freebase" is the base form of cocaine, as opposed to the salt form. It is practically insoluble in water whereas hydrochloride salt is water-soluble.
Smoking freebase cocaine has the additional effect of releasing methylecgonidine into the user's system due to the pyrolysis of the substance (a side effect which insufflating or injecting powder cocaine does not create). Some research suggests that smoking freebase cocaine can be even more cardiotoxic than other routes of administration[123] because of methylecgonidine's effects on lung tissue[124] and liver tissue.[125]
Pure cocaine is prepared by neutralizing its compounding salt with an alkaline solution, which will precipitate non-polar basic cocaine. It is further refined through aqueous-solvent liquid–liquid extraction.
Crack cocaine

Crack is usually smoked in a glass pipe, and once inhaled, it passes from the lungs directly to the central nervous system, producing an almost immediate "high" that can be very powerful – this initial crescendo of stimulation is known as a "rush". This is followed by an equally intense low, leaving the user craving more drug. Addiction to crack usually occurs with four to six weeks; much more rapidly than with regular cocaine.[126]
Powder cocaine (cocaine hydrochloride) must be heated to a high temperature (about 197 °C), and considerable decomposition/burning occurs at these high temperatures. This effectively destroys some of the cocaine and yields a sharp, acrid, and foul-tasting smoke. Cocaine base/crack can be smoked because it vaporizes with little or no decomposition at 98 °C (208 °F),[127] which is below the boiling point of water.
Crack is a lower purity form of free-base cocaine that is usually produced by neutralization of cocaine hydrochloride with a solution of baking soda (sodium bicarbonate, NaHCO3) and water, producing a very hard/brittle, off-white-to-brown colored, amorphous material that contains sodium carbonate, entrapped water, and other by-products as the main impurities. The origin of the name "crack" comes from the "crackling" sound (and hence the onomatopoeic moniker "crack") that is produced when the cocaine and its impurities (i.e. water, sodium bicarbonate) are heated past the point of vaporization.[128]
Coca leaf infusions
Coca herbal infusion (also referred to as coca tea) is used in coca-leaf producing countries much as any herbal medicinal infusion would elsewhere in the world. The free and legal commercialization of dried coca leaves under the form of filtration bags to be used as "coca tea" has been actively promoted by the governments of Peru and Bolivia for many years as a drink having medicinal powers. In Peru, the National Coca Company, a state-run corporation, sells cocaine-infused teas and other medicinal products and also exports leaves to the U.S. for medicinal use.[129]
Visitors to the city of Cuzco in Peru, and La Paz in Bolivia are greeted with the offering of coca leaf infusions (prepared in teapots with whole coca leaves) purportedly to help the newly arrived traveler overcome the malaise of high altitude sickness.[130] The effects of drinking coca tea are mild stimulation and mood lift.[131] It has also been promoted as an adjuvant for the treatment of cocaine dependence. One study on coca leaf infusion used with counseling in the treatment of 23 addicted coca-paste smokers in Lima, Peru found that the relapses rate fell from 4.35 times per month on average before coca tea treatment to one during treatment. The duration of abstinence increased from an average of 32 days before treatment to 217.2 days during treatment. This suggests that coca leaf infusion plus counseling may be effective at preventing relapse during cocaine addiction treatment.[132]
There is little information on the pharmacological and toxicological effects of consuming coca tea. A chemical analysis by solid-phase extraction and gas chromatography–mass spectrometry (SPE-GC/MS) of Peruvian and Bolivian tea bags indicated the presence of significant amounts of cocaine, the metabolite benzoylecgonine, ecgonine methyl ester and trans-cinnamoylcocaine in coca tea bags and coca tea. Urine specimens were also analyzed from an individual who consumed one cup of coca tea and it was determined that enough cocaine and cocaine-related metabolites were present to produce a positive drug test.[133]
Synthesis
Biosynthesis
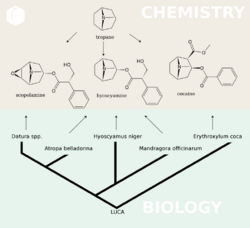
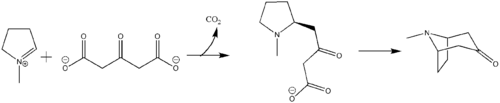
The first synthesis and elucidation of the cocaine molecule was by Richard Willstätter in 1898.[134] Willstätter's synthesis derived cocaine from tropinone. Since then, Robert Robinson and Edward Leete have made significant contributions to the mechanism of the synthesis. (-NO3)
The additional carbon atoms required for the synthesis of cocaine are derived from acetyl-CoA, by addition of two acetyl-CoA units to the N-methyl-Δ1-pyrrolinium cation.[135] The first addition is a Mannich-like reaction with the enolate anion from acetyl-CoA acting as a nucleophile towards the pyrrolinium cation. The second addition occurs through a Claisen condensation. This produces a racemic mixture of the 2-substituted pyrrolidine, with the retention of the thioester from the Claisen condensation. In formation of tropinone from racemic ethyl [2,3-13C2]4(Nmethyl-2-pyrrolidinyl)-3-oxobutanoate there is no preference for either stereoisomer.[136] In cocaine biosynthesis, only the (S)-enantiomer can cyclize to form the tropane ring system of cocaine. The stereoselectivity of this reaction was further investigated through study of prochiral methylene hydrogen discrimination.[137] This is due to the extra chiral center at C-2.[138] This process occurs through an oxidation, which regenerates the pyrrolinium cation and formation of an enolate anion, and an intramolecular Mannich reaction. The tropane ring system undergoes hydrolysis, SAM-dependent methylation, and reduction via NADPH for the formation of methylecgonine. The benzoyl moiety required for the formation of the cocaine diester is synthesized from phenylalanine via cinnamic acid.[139] Benzoyl-CoA then combines the two units to form cocaine.
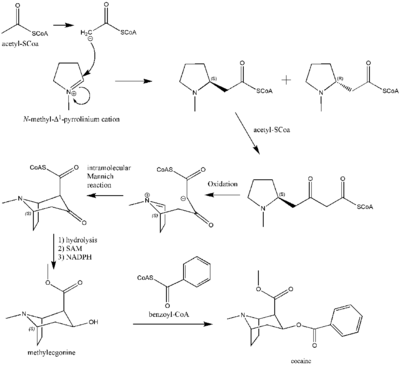
N-methyl-pyrrolinium cation
The biosynthesis begins with L-Glutamine, which is derived to L-ornithine in plants. The major contribution of L-ornithine and L-arginine as a precursor to the tropane ring was confirmed by Edward Leete.[140] Ornithine then undergoes a pyridoxal phosphate-dependent decarboxylation to form putrescine. In some animals, the urea cycle derives putrescine from ornithine. L-ornithine is converted to L-arginine,[141] which is then decarboxylated via PLP to form agmatine. Hydrolysis of the imine derives N-carbamoylputrescine followed with hydrolysis of the urea to form putrescine. The separate pathways of converting ornithine to putrescine in plants and animals have converged. A SAM-dependent N-methylation of putrescine gives the N-methylputrescine product, which then undergoes oxidative deamination by the action of diamine oxidase to yield the aminoaldehyde. Schiff base formation confirms the biosynthesis of the N-methyl-Δ1-pyrrolinium cation.
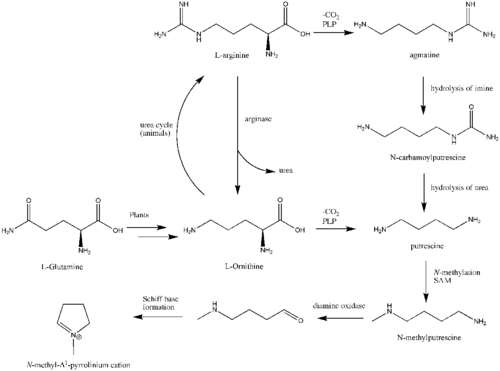
Robert Robinson's acetonedicarboxylate
The biosynthesis of the tropane alkaloid is still not understood. Hemscheidt proposes that Robinson's acetonedicarboxylate emerges as a potential intermediate for this reaction.[142] Condensation of N-methylpyrrolinium and acetonedicarboxylate would generate the oxobutyrate. Decarboxylation leads to tropane alkaloid formation.
Reduction of tropinone
The reduction of tropinone is mediated by NADPH-dependent reductase enzymes, which have been characterized in multiple plant species.[143] These plant species all contain two types of the reductase enzymes, tropinone reductase I and tropinone reductase II. TRI produces tropine and TRII produces pseudotropine. Due to differing kinetic and pH/activity characteristics of the enzymes and by the 25-fold higher activity of TRI over TRII, the majority of the tropinone reduction is from TRI to form tropine.[144]
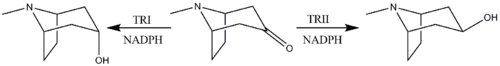
GMO synthesis
Research
In 2022, a GMO produced N. benthamiana were discovered that were able to produce 25% of the amount of cocaine found in a coca plant.[145]
Detection in body fluids
Cocaine and its major metabolites may be quantified in blood, plasma, or urine to monitor for use, confirm a diagnosis of poisoning, or assist in the forensic investigation of a traffic or other criminal violation or sudden death. Most commercial cocaine immunoassay screening tests cross-react appreciably with the major cocaine metabolites, but chromatographic techniques can easily distinguish and separately measure each of these substances. When interpreting the results of a test, it is important to consider the cocaine usage history of the individual, since a chronic user can develop tolerance to doses that would incapacitate a cocaine-naive individual, and the chronic user often has high baseline values of the metabolites in his system. Cautious interpretation of testing results may allow a distinction between passive or active usage, and between smoking versus other routes of administration.[146]
Field analysis
Cocaine may be detected by law enforcement using the Scott reagent. The test can easily generate false positives for common substances and must be confirmed with a laboratory test.[147][148]
Approximate cocaine purity can be determined using 1 mL 2% cupric sulfate pentahydrate in dilute HCl, 1 mL 2% potassium thiocyanate and 2 mL of chloroform. The shade of brown shown by the chloroform is proportional to the cocaine content. This test is not cross sensitive to heroin, methamphetamine, benzocaine, procaine and a number of other drugs but other chemicals could cause false positives.[149]
Usage
| Substance | Best estimate |
Low estimate |
High estimate |
|---|---|---|---|
| Amphetamine- type stimulants |
34.16 | 13.42 | 55.24 |
| Cannabis | 192.15 | 165.76 | 234.06 |
| Cocaine | 18.20 | 13.87 | 22.85 |
| Ecstasy | 20.57 | 8.99 | 32.34 |
| Opiates | 19.38 | 13.80 | 26.15 |
| Opioids | 34.26 | 27.01 | 44.54 |
According to a 2016 United Nations report, England and Wales are the countries with the highest rate of cocaine usage (2.4% of adults in the previous year).[151] Other countries where the usage rate meets or exceeds 1.5% are Spain and Scotland (2.2%), the United States (2.1%), Australia (2.1%), Uruguay (1.8%), Brazil (1.75%), Chile (1.73%), the Netherlands (1.5%) and Ireland (1.5%).[151]
Europe
Cocaine is the second most popular illegal recreational drug in Europe (behind cannabis). Since the mid-1990s, overall cocaine usage in Europe has been on the rise, but usage rates and attitudes tend to vary between countries. European countries with the highest usage rates are the United Kingdom, Spain, Italy, and the Republic of Ireland.
Approximately 17 million Europeans (5.1%) have used cocaine at least once and 3.5 million (1.1%) in the last year. About 1.9% (2.3 million) of young adults (15–34 years old) have used cocaine in the last year (latest data available as of 2018).[152]
Usage is particularly prevalent among this demographic: 4% to 7% of males have used cocaine in the last year in Spain, Denmark, the Republic of Ireland, Italy, and the United Kingdom. The ratio of male to female users is approximately 3.8:1, but this statistic varies from 1:1 to 13:1 depending on country.[153]
In 2014 London had the highest amount of cocaine in its sewage out of 50 European cities.[154]
United States
Cocaine is the second most popular illegal recreational drug in the United States (behind cannabis)[155] and the U.S. is the world's largest consumer of cocaine.[156] Its users span over different ages, races, and professions. In the 1970s and 1980s, the drug became particularly popular in the disco culture as cocaine usage was very common and popular in many discos such as Studio 54.
Dependence treatment
History
Discovery

Indigenous peoples of South America have chewed the leaves of Erythroxylon coca—a plant that contains vital nutrients as well as numerous alkaloids, including cocaine—for over a thousand years.[157] The coca leaf was, and still is, chewed almost universally by some indigenous communities. The remains of coca leaves have been found with ancient Peruvian mummies, and pottery from the time period depicts humans with bulged cheeks, indicating the presence of something on which they are chewing.[158] There is also evidence that these cultures used a mixture of coca leaves and saliva as an anesthetic for the performance of trepanation.[159]
When the Spanish arrived in South America, the conquistadors at first banned coca as an "evil agent of devil". But after discovering that without the coca the locals were barely able to work, the conquistadors legalized and taxed the leaf, taking 10% off the value of each crop.[160] In 1569, Spanish botanist Nicolás Monardes described the indigenous peoples' practice of chewing a mixture of tobacco and coca leaves to induce "great contentment":
When they wished to make themselves drunk and out of judgment they chewed a mixture of tobacco and coca leaves which make them go as they were out of their wittes.[161]
In 1609, Padre Blas Valera wrote:
Coca protects the body from many ailments, and our doctors use it in powdered form to reduce the swelling of wounds, to strengthen broken bones, to expel cold from the body or prevent it from entering, and to cure rotten wounds or sores that are full of maggots. And if it does so much for outward ailments, will not its singular virtue have even greater effect in the entrails of those who eat it?[162]
Isolation and naming
Although the stimulant and hunger-suppressant properties of coca had been known for many centuries, the isolation of the cocaine alkaloid was not achieved until 1855. Various European scientists had attempted to isolate cocaine, but none had been successful for two reasons: the knowledge of chemistry required was insufficient at the time, and contemporary conditions of sea-shipping from South America could degrade the cocaine in the plant samples available to European chemists.[citation needed]
The cocaine alkaloid was first isolated by the German chemist Friedrich Gaedcke in 1855. Gaedcke named the alkaloid "erythroxyline", and published a description in the journal Archiv der Pharmazie.[163]
In 1856, Friedrich Wöhler asked Dr. Carl Scherzer, a scientist aboard the Novara (an Austrian frigate sent by Emperor Franz Joseph to circle the globe), to bring him a large amount of coca leaves from South America. In 1859, the ship finished its travels and Wöhler received a trunk full of coca. Wöhler passed on the leaves to Albert Niemann, a PhD student at the University of Göttingen in Germany, who then developed an improved purification process.[164]
Niemann described every step he took to isolate cocaine in his dissertation titled Über eine neue organische Base in den Cocablättern (On a New Organic Base in the Coca Leaves), which was published in 1860 and earned him his Ph.D. He wrote of the alkaloid's "colourless transparent prisms" and said that "Its solutions have an alkaline reaction, a bitter taste, promote the flow of saliva and leave a peculiar numbness, followed by a sense of cold when applied to the tongue." Niemann named the alkaloid "cocaine" from "coca" (from Quechua "kúka") + suffix "ine".[164][165]
The first synthesis and elucidation of the structure of the cocaine molecule was by Richard Willstätter in 1898.[134] It was the first biomimetic synthesis of an organic structure recorded in academic chemical literature.[166][167] The synthesis started from tropinone, a related natural product and took five steps.
Because of the former use of cocaine as a local anesthetic, a suffix "-caine" was later extracted and used to form names of synthetic local anesthetics.
Medicalization



With the discovery of this new alkaloid, Western medicine was quick to exploit the possible uses of this plant.
In 1879, Vassili von Anrep, of the University of Würzburg, devised an experiment to demonstrate the analgesic properties of the newly discovered alkaloid. He prepared two separate jars, one containing a cocaine-salt solution, with the other containing merely saltwater. He then submerged a frog's legs into the two jars, one leg in the treatment and one in the control solution, and proceeded to stimulate the legs in several different ways. The leg that had been immersed in the cocaine solution reacted very differently from the leg that had been immersed in saltwater.[168]
Karl Koller (a close associate of Sigmund Freud, who would write about cocaine later) experimented with cocaine for ophthalmic usage. In an infamous experiment in 1884, he experimented upon himself by applying a cocaine solution to his own eye and then pricking it with pins. His findings were presented to the Heidelberg Ophthalmological Society. Also in 1884, Jellinek demonstrated the effects of cocaine as a respiratory system anesthetic. In 1885, William Halsted demonstrated nerve-block anesthesia,[169] and James Leonard Corning demonstrated peridural anesthesia.[170] 1898 saw Heinrich Quincke use cocaine for spinal anesthesia.
Popularization

In 1859, an Italian doctor, Paolo Mantegazza, returned from Peru, where he had witnessed first-hand the use of coca by the local indigenous peoples. He proceeded to experiment on himself and upon his return to Milan, he wrote a paper in which he described the effects. In this paper, he declared coca and cocaine (at the time they were assumed to be the same) as being useful medicinally, in the treatment of "a furred tongue in the morning, flatulence, and whitening of the teeth."
A chemist named Angelo Mariani who read Mantegazza's paper became immediately intrigued with coca and its economic potential. In 1863, Mariani started marketing a wine called Vin Mariani, which had been treated with coca leaves, to become coca wine. The ethanol in wine acted as a solvent and extracted the cocaine from the coca leaves, altering the drink's effect. It contained 6 mg cocaine per ounce of wine, but Vin Mariani which was to be exported contained 7.2 mg per ounce, to compete with the higher cocaine content of similar drinks in the United States. A "pinch of coca leaves" was included in John Styth Pemberton's original 1886 recipe for Coca-Cola, though the company began using decocainized leaves in 1906 when the Pure Food and Drug Act was passed.
In 1879 cocaine began to be used to treat morphine addiction. Cocaine was introduced into clinical use as a local anesthetic in Germany in 1884, about the same time as Sigmund Freud published his work Über Coca,[172] in which he wrote that cocaine causes:[173]
Exhilaration and lasting euphoria, which in no way differs from the normal euphoria of the healthy person. You perceive an increase of self-control and possess more vitality and capacity for work. In other words, you are simply normal, and it is soon hard to believe you are under the influence of any drug. Long intensive physical work is performed without any fatigue. This result is enjoyed without any of the unpleasant after-effects that follow exhilaration brought about by alcoholic beverages. No craving for the further use of cocaine appears after the first, or even after repeated taking of the drug.[174]
By 1885 the U.S. manufacturer Parke-Davis sold coca-leaf cigarettes and cheroots, a cocaine inhalant, a Coca Cordial, cocaine crystals, and cocaine solution for intravenous injection.[175] The company promised that its cocaine products would "supply the place of food, make the coward brave, the silent eloquent and render the sufferer insensitive to pain."

By the late Victorian era, cocaine use had appeared as a vice in literature. For example, it was injected by Arthur Conan Doyle's fictional Sherlock Holmes, generally to offset the boredom he felt when he was not working on a case.
In early 20th-century Memphis, Tennessee, cocaine was sold in neighborhood drugstores on Beale Street, costing five or ten cents for a small boxful. Stevedores along the Mississippi River used the drug as a stimulant, and white employers encouraged its use by black laborers.[176]
In 1909, Ernest Shackleton took "Forced March" brand cocaine tablets to Antarctica, as did Captain Scott a year later on his ill-fated journey to the South Pole.[177]
In the 1931 song "Minnie the Moocher", Cab Calloway heavily references cocaine use. He uses the phrase "kicking the gong around", slang for cocaine use; describes titular character Minnie as "tall and skinny;" and describes Smokey Joe as "cokey".[178] In the 1932 comedy musical film The Big Broadcast, Cab Calloway performs the song with his orchestra and mimes snorting cocaine in between verses.[179]
During the mid-1940s, amidst World War II, cocaine was considered for inclusion as an ingredient of a future generation of 'pep pills' for the German military, code named D-IX.[180]
In modern popular culture, references to cocaine are common. The drug has a glamorous image associated with the wealthy, famous and powerful, and is said to make users "feel rich and beautiful".[181][182][183][184] In addition the pace of modern society − such as in finance − gives many the incentive to make use of the drug.[181]

Modern usage

In many countries, cocaine is a popular recreational drug. In the United States, the development of "crack" cocaine introduced the substance to a generally poorer inner-city market. The use of the powder form has stayed relatively constant, experiencing a new height of use during the late 1990s and early 2000s in the U.S., and has become much more popular in the last few years in the UK. [citation needed][when?]
Cocaine use is prevalent across all socioeconomic strata, including age, demographics, economic, social, political, religious, and livelihood.[185]
The estimated U.S. cocaine market exceeded US$70 billion in street value for the year 2005, exceeding revenues by corporations such as Starbucks.[186][187] Cocaine's status as a club drug shows its immense popularity among the "party crowd".[185]
In 1995 the World Health Organization (WHO) and the United Nations Interregional Crime and Justice Research Institute (UNICRI) announced in a press release the publication of the results of the largest global study on cocaine use ever undertaken. An American representative in the World Health Assembly banned the publication of the study, because it seemed to make a case for the positive uses of cocaine. An excerpt of the report strongly conflicted with accepted paradigms, for example, "that occasional cocaine use does not typically lead to severe or even minor physical or social problems." In the sixth meeting of the B committee, the US representative threatened that "If World Health Organization activities relating to drugs failed to reinforce proven drug control approaches, funds for the relevant programs should be curtailed". This led to the decision to discontinue publication. A part of the study was recuperated and published in 2010, including profiles of cocaine use in 20 countries, but are unavailable as of 2015[update].[188]
In October 2010 it was reported that the use of cocaine in Australia has doubled since monitoring began in 2003.[189]
A problem with illegal cocaine use, especially in the higher volumes used to combat fatigue (rather than increase euphoria) by long-term users, is the risk of ill effects or damage caused by the compounds used in adulteration. Cutting or "stepping on" the drug is commonplace, using compounds which simulate ingestion effects, such as Novocain (procaine) producing temporary anesthesia, as many users believe a strong numbing effect is the result of strong and/or pure cocaine, ephedrine or similar stimulants that are to produce an increased heart rate. The normal adulterants for profit are inactive sugars, usually mannitol, creatine, or glucose, so introducing active adulterants gives the illusion of purity and to 'stretch' or make it so a dealer can sell more product than without the adulterants.[citation needed] The adulterant of sugars allows the dealer to sell the product for a higher price because of the illusion of purity and allows the sale of more of the product at that higher price, enabling dealers to significantly increase revenue with little additional cost for the adulterants. A 2007 study by the European Monitoring Centre for Drugs and Drug Addiction showed that the purity levels for street purchased cocaine was often under 5% and on average under 50% pure.[190]
Society and culture
Legal status
The production, distribution, and sale of cocaine products is restricted (and illegal in most contexts) in most countries as regulated by the Single Convention on Narcotic Drugs, and the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances. In the United States the manufacture, importation, possession, and distribution of cocaine are additionally regulated by the 1970 Controlled Substances Act.
Some countries, such as Peru and Bolivia, permit the cultivation of coca leaf for traditional consumption by the local indigenous population, but nevertheless, prohibit the production, sale, and consumption of cocaine.[191] The provisions as to how much a coca farmer can yield annually is protected by laws such as the Bolivian Cato accord.[192] In addition, some parts of Europe, the United States, and Australia allow processed cocaine for medicinal uses only.
Australia
Cocaine is a Schedule 8 controlled drug in Australia under the Poisons Standard.[193] It is the second most popular illicit recreational drug in Australia behind cannabis.[194]
In Western Australia under the Misuse of Drugs Act 1981 4.0g of cocaine is the amount of prohibited drugs determining a court of trial, 2.0g is the amount of cocaine required for the presumption of intention to sell or supply and 28.0g is the amount of cocaine required for purposes of drug trafficking.[195]
United States
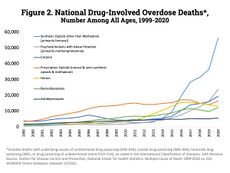
The US federal government instituted a national labeling requirement for cocaine and cocaine-containing products through the Pure Food and Drug Act of 1906.[196] The next important federal regulation was the Harrison Narcotics Tax Act of 1914. While this act is often seen as the start of prohibition, the act itself was not actually a prohibition on cocaine, but instead set up a regulatory and licensing regime.[197] The Harrison Act did not recognize addiction as a treatable condition and therefore the therapeutic use of cocaine, heroin, or morphine to such individuals was outlawed – leading a 1915 editorial in the journal American Medicine to remark that the addict "is denied the medical care he urgently needs, open, above-board sources from which he formerly obtained his drug supply are closed to him, and he is driven to the underworld where he can get his drug, but of course, surreptitiously and in violation of the law."[198] The Harrison Act left manufacturers of cocaine untouched so long as they met certain purity and labeling standards.[199] Despite that cocaine was typically illegal to sell and legal outlets were rarer, the quantities of legal cocaine produced declined very little.[199] Legal cocaine quantities did not decrease until the Jones–Miller Act of 1922 put serious restrictions on cocaine manufactures.[199]
Before the early 1900s, the primary problem caused by cocaine use was portrayed by newspapers to be addiction, not violence or crime, and the cocaine user was represented as an upper- or middle-class White person. In 1914, The New York Times published an article titled "Negro Cocaine 'Fiends' Are a New Southern Menace", portraying Black cocaine users as dangerous and able to withstand wounds that would normally be fatal.[200] The Anti-Drug Abuse Act of 1986 mandated prison sentences for 500 grams of powdered cocaine and 5 grams of crack cocaine.[201] In the National Survey on Drug Use and Health, Whites reported a higher rate of powdered cocaine use, and Blacks reported a higher rate of crack cocaine use.[202]
Interdiction


In 2004, according to the United Nations , 589 tonnes of cocaine were seized globally by law enforcement authorities. Colombia seized 188 t, the United States 166 t, Europe 79 t, Peru 14 t, Bolivia 9 t, and the rest of the world 133 t.[203]
Production
Colombia is as of 2019 the world's largest cocaine producer, with production more than tripling since 2013.[204][205] Three-quarters of the world's annual yield of cocaine has been produced in Colombia, both from cocaine base imported from Peru (primarily the Huallaga Valley) and Bolivia and from locally grown coca. There was a 28% increase in the amount of potentially harvestable coca plants which were grown in Colombia in 1998. This, combined with crop reductions in Bolivia and Peru, made Colombia the nation with the largest area of coca under cultivation after the mid-1990s. Coca grown for traditional purposes by indigenous communities, a use which is still present and is permitted by Colombian laws, only makes up a small fragment of total coca production, most of which is used for the illegal drug trade.[citation needed]
An interview with a coca farmer published in 2003 described a mode of production by acid-base extraction that has changed little since 1905. Roughly 625 pounds (283 kg) of leaves were harvested per hectare, six times per year. The leaves were dried for half a day, then chopped into small pieces with a string trimmer and sprinkled with a small amount of powdered cement (replacing sodium carbonate from former times). Several hundred pounds of this mixture were soaked in 50 US gallons (190 L) of gasoline for a day, then the gasoline was removed and the leaves were pressed for the remaining liquid, after which they could be discarded. Then battery acid (weak sulfuric acid) was used, one bucket per 55 lb (25 kg) of leaves, to create a phase separation in which the cocaine free base in the gasoline was acidified and extracted into a few buckets of "murky-looking smelly liquid". Once powdered caustic soda was added to this, the cocaine precipitated and could be removed by filtration through a cloth. The resulting material, when dried, was termed pasta and sold by the farmer. The 3,750 pounds (1,700 kg) yearly harvest of leaves from a hectare produced 6 lb (2.5 kg) of pasta, approximately 40–60% cocaine. Repeated recrystallization from solvents, producing pasta lavada and eventually crystalline cocaine were performed at specialized laboratories after the sale.[206]
Attempts to eradicate coca fields through the use of defoliants have devastated part of the farming economy in some coca-growing regions of Colombia, and strains appear to have been developed that are more resistant or immune to their use. Whether these strains are natural mutations or the product of human tampering is unclear. These strains have also shown to be more potent than those previously grown, increasing profits for the drug cartels responsible for the exporting of cocaine. Although production fell temporarily, coca crops rebounded in numerous smaller fields in Colombia, rather than the larger plantations.[citation needed]
The cultivation of coca has become an attractive economic decision for many growers due to the combination of several factors, including the lack of other employment alternatives, the lower profitability of alternative crops in official crop substitution programs, the eradication-related damages to non-drug farms, the spread of new strains of the coca plant due to persistent worldwide demand.[citation needed]
| 2000 | 2001 | 2002 | 2003 | 2004 | |
|---|---|---|---|---|---|
| Net cultivation km2 (sq mi) | 1,875 (724) | 2,218 (856) | 2,007.5 (775.1) | 1,663 (642) | 1,662 (642) |
| Potential pure cocaine production (tonnes) | 770 | 925 | 830 | 680 | 645 |
The latest estimate provided by the U.S. authorities on the annual production of cocaine in Colombia refers to 290 metric tons. As of the end of 2011, the seizure operations of Colombian cocaine carried out in different countries have totaled 351.8 metric tons of cocaine, i.e. 121.3% of Colombia's annual production according to the U.S. Department of State's estimates.[208][209]
Synthesis
Synthesizing cocaine could eliminate the high visibility and low reliability of offshore sources and international smuggling, replacing them with clandestine domestic laboratories, as are common for illicit methamphetamine, but is rarely done. Natural cocaine remains the lowest cost and highest quality supply of cocaine. Formation of inactive stereoisomers (cocaine has four chiral centres – 1R 2R, 3S, and 5S, two of them dependent, hence eight possible stereoisomers) plus synthetic by-products limits the yield and purity.[citation needed]
Trafficking and distribution

Organized criminal gangs operating on a large scale dominate the cocaine trade. Most cocaine is grown and processed in South America, particularly in Colombia, Bolivia, Peru, and smuggled into the United States and Europe, the United States being the world's largest consumer of cocaine,[156] where it is sold at huge markups; usually in the US at $80–120 for 1 gram, and $250–300 for 3.5 grams (1/8 of an ounce, or an "eight ball").[210]
Caribbean and Mexican routes
The primary cocaine importation points in the United States have been in Arizona, southern California , southern Florida, and Texas . Typically, land vehicles are driven across the U.S.–Mexico border. Sixty-five percent of cocaine enters the United States through Mexico, and the vast majority of the rest enters through Florida.[211][page needed] As of 2015[update], the Sinaloa Cartel is the most active drug cartel involved in smuggling illicit drugs like cocaine into the United States and trafficking them throughout the United States.[212]
Cocaine traffickers from Colombia and Mexico have established a labyrinth of smuggling routes throughout the Caribbean, the Bahama Island chain, and South Florida. They often hire traffickers from Mexico or the Dominican Republic to transport the drug using a variety of smuggling techniques to U.S. markets. These include airdrops of 500 to 700 kg (1,100 to 1,500 lb) in the Bahama Islands or off the coast of Puerto Rico, mid-ocean boat-to-boat transfers of 500 to 2,000 kg (1,100 to 4,400 lb), and the commercial shipment of tonnes of cocaine through the port of Miami.[citation needed]
Chilean route
Another route of cocaine traffic goes through Chile, which is primarily used for cocaine produced in Bolivia since the nearest seaports lie in northern Chile. The arid Bolivia–Chile border is easily crossed by 4×4 vehicles that then head to the seaports of Iquique and Antofagasta. While the price of cocaine is higher in Chile than in Peru and Bolivia, the final destination is usually Europe, especially Spain where drug dealing networks exist among South American immigrants.[citation needed]
Techniques
Cocaine is also carried in small, concealed, kilogram quantities across the border by couriers known as "mules" (or "mulas"), who cross a border either legally, for example, through a port or airport, or illegally elsewhere. The drugs may be strapped to the waist or legs or hidden in bags, or hidden in the body. If the mule gets through without being caught, the gangs will reap most of the profits. If caught, gangs will sever all links and the mule will usually stand trial for trafficking alone.[citation needed]
Bulk cargo ships are also used to smuggle cocaine to staging sites in the western Caribbean–Gulf of Mexico area. These vessels are typically 150–250-foot (50–80 m) coastal freighters that carry an average cocaine load of approximately 2.5 tonnes. Commercial fishing vessels are also used for smuggling operations. In areas with a high volume of recreational traffic, smugglers use the same types of vessels, such as go-fast boats, like those used by the local populations.[citation needed]
Sophisticated drug subs are the latest tool drug runners are using to bring cocaine north from Colombia, it was reported on 20 March 2008. Although the vessels were once viewed as a quirky sideshow in the drug war, they are becoming faster, more seaworthy, and capable of carrying bigger loads of drugs than earlier models, according to those charged with catching them.[213]
Sales to consumers

Cocaine is readily available in all major countries' metropolitan areas. According to the Summer 1998 Pulse Check, published by the U.S. Office of National Drug Control Policy, cocaine use had stabilized across the country, with a few increases reported in San Diego, Bridgeport, Miami, and Boston. In the West, cocaine usage was lower, which was thought to be due to a switch to methamphetamine among some users; methamphetamine is cheaper, three and a half times more powerful, and lasts 12–24 times longer with each dose.[214][215] Nevertheless, the number of cocaine users remain high, with a large concentration among urban youth.
In addition to the amounts previously mentioned, cocaine can be sold in "bill sizes": As of 2007[update] for example, $10 might purchase a "dime bag", a very small amount (0.1–0.15 g) of cocaine. These amounts and prices are very popular among young people because they are inexpensive and easily concealed on one's body. Quality and price can vary dramatically depending on supply and demand, and on geographic region.[216]
In 2008, the European Monitoring Centre for Drugs and Drug Addiction reports that the typical retail price of cocaine varied between €50 and €75 per gram in most European countries, although Cyprus, Romania, Sweden, and Turkey reported much higher values.[217]
Consumption
World annual cocaine consumption, as of 2000, stood at around 600 tonnes, with the United States consuming around 300 t, 50% of the total, Europe about 150 t, 25% of the total, and the rest of the world the remaining 150 t or 25%.[218] It is estimated that 1.5 million people in the United States used cocaine in 2010, down from 2.4 million in 2006.[13] Conversely, cocaine use appears to be increasing[when?] in Europe with the highest prevalences in Spain , the United Kingdom , Italy, and Ireland.[13]
The 2010 UN World Drug Report concluded that "it appears that the North American cocaine market has declined in value from US$47 billion in 1998 to US$38 billion in 2008. Between 2006 and 2008, the value of the market remained basically stable".[219]
See also
- Black cocaine
- Coca alkaloids
- Coca eradication
- Cocaine and amphetamine regulated transcript
- Cocaine Anonymous
- Cocaine Cowboys
- Cocaine paste
- Crack cocaine § Crack lung
- Crack epidemic
- Illegal drug trade in Latin America
- Coca production in Colombia
- Clan del Golfo
- Legal status of cocaine
- List of cocaine analogues
- List of countries by prevalence of cocaine use
- Methylphenidate
- Modafinil
- Pre-Columbian trans-oceanic evidence for cocaine in ancient Egypt
- Prenatal cocaine exposure
- Route 36, cocaine bar in Bolivia
- Tranquilandia
- Ypadu
References
- ↑ The A-Z Encyclopedia of Alcohol and Drug Abuse. Universal-Publishers. 2002. p. 461. ISBN 978-1-58112-404-0. https://books.google.com/books?id=4yaGePenGKgC&pg=PA461.
- ↑ 2.0 2.1 2.2 "Goprelto – cocaine hydrochloride solution". 3 January 2020. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=689750b7-8e51-47d9-a428-078f3f6c9dec.
- ↑ 3.0 3.1 3.2 "Numbrino – cocaine hydrochloride nasal solution". 28 February 2020. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94f9b3f8-bce5-41ed-9453-c54ed1d6c269.
- ↑ "Cocaine topical (C-Topical Solution) Use During Pregnancy". 10 April 2020. https://www.drugs.com/pregnancy/cocaine-topical.html.
- ↑ Ghodse's Drugs and Addictive Behaviour: A Guide to Treatment (4 ed.). Cambridge University Press. 2010. p. 91. ISBN 978-1-139-48567-8. https://books.google.com/books?id=WYQ23OMjWbcC&pg=PA91.
- ↑ Introduction to Pharmacology (3 ed.). Abingdon: CRC Press. 2007. pp. 222–223. ISBN 978-1-4200-4742-4. https://books.google.com/books?id=qfrLBQAAQBAJ&pg=PA222.
- ↑ 7.0 7.1 "Monoamines: Dopamine, Norepinephrine, and Serotonin, Beyond Modulation, "Switches" That Alter the State of Target Networks". The Neuroscientist 28 (2): 121–143. December 2020. doi:10.1177/1073858420974336. PMID 33292070.
- ↑ "DEA / Drug Scheduling". https://www.dea.gov/druginfo/ds.shtml.
- ↑ 9.0 9.1 "Nasal mucosal versus gastrointestinal absorption of nasally administered cocaine". European Journal of Clinical Pharmacology 56 (4): 305–10. July 2000. doi:10.1007/s002280000147. PMID 10954344.
- ↑ "Cocaine pharmacokinetics in humans". Journal of Ethnopharmacology 3 (2–3): 353–66. 1981. doi:10.1016/0378-8741(81)90063-5. PMID 7242115.
- ↑ "Cocaine disposition in humans after intravenous injection, nasal insufflation (snorting), or smoking". Drug Metabolism and Disposition 17 (2): 153–9. 1989. PMID 2565204.
- ↑ 12.0 12.1 "Intranasal and oral cocaine kinetics". Clinical Pharmacology and Therapeutics 27 (3): 386–94. March 1980. doi:10.1038/clpt.1980.52. PMID 7357795.
- ↑ 13.00 13.01 13.02 13.03 13.04 13.05 13.06 13.07 13.08 13.09 13.10 13.11 13.12 13.13 13.14 13.15 13.16 13.17 13.18 13.19 "Cocaine intoxication". Critical Care Clinics 28 (4): 517–26. October 2012. doi:10.1016/j.ccc.2012.07.003. PMID 22998988.
- ↑ "Cocaine". https://ahdictionary.com/word/search.html?q=+COCAINE.
- ↑ "The identification of coca (Erythroxylum species): 1860–1910". Botanical Journal of the Linnean Society 84 (4): 329–353. June 1982. doi:10.1111/j.1095-8339.1982.tb00368.x.
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 "Data available on the extent of cocaine use and dependence: biochemistry, pharmacologic effects and global burden of disease of cocaine abusers". Current Medicinal Chemistry 19 (33): 5647–57. 2012. doi:10.2174/092986712803988811. PMID 22856655.
- ↑ "Experimental treatments for cocaine toxicity: a difficult transition to the bedside". The Journal of Pharmacology and Experimental Therapeutics 347 (2): 251–7. November 2013. doi:10.1124/jpet.113.206383. PMID 23978563.
- ↑ "Topical Anesthesia in Office-Based Laryngeal Surgery". Office-Based Laryngeal Surgery. USA: Springer. 2022. pp. 123–137. doi:10.1007/978-3-030-91936-8_6. ISBN 978-3-030-91935-1. https://link.springer.com/chapter/10.1007/978-3-030-91936-8_6.
- ↑ 19.0 19.1 "How do psychostimulants enter the human brain? Analysis of the role of the proton-organic cation antiporter". Biochemical Pharmacology 192: 114751. October 2021. doi:10.1016/j.bcp.2021.114751. PMID 34464621.
- ↑ 20.0 20.1 "Structural Requirements for Uptake of Diphenhydramine Analogs into hCMEC/D3 Cells Via the Proton-Coupled Organic Cation Antiporter". Journal of Pharmaceutical Sciences 110 (1): 397–403. January 2021. doi:10.1016/j.xphs.2020.09.001. PMID 32898521.
- ↑ 21.0 21.1 "Carrier-mediated cocaine transport at the blood–brain barrier as a putative mechanism in addiction liability". The International Journal of Neuropsychopharmacology 18 (1): pyu001. October 2014. doi:10.1093/ijnp/pyu001. PMID 25539501.
- ↑ "Insights into the Modulation of Dopamine Transporter Function by Amphetamine, Orphenadrine, and Cocaine Binding". Frontiers in Neurology 6: 134. 2015. doi:10.3389/fneur.2015.00134. PMID 26106364.
- ↑ "Effects of stimulant drug use on the dopaminergic system: A systematic review and meta-analysis of in vivo neuroimaging studies". European Psychiatry 59: 15–24. June 2019. doi:10.1016/j.eurpsy.2019.03.003. PMID 30981746.
- ↑ "How does cocaine produce its effects?". https://www.drugabuse.gov/publications/research-reports/cocaine/how-does-cocaine-produce-its-effects.
- ↑ "Dopamine and Addiction". Annual Review of Psychology 71 (1): 79–106. January 2020. doi:10.1146/annurev-psych-010418-103337. PMID 31905114.
- ↑ 26.0 26.1 "Acute tolerance to cocaine in humans". Clinical Pharmacology and Therapeutics 44 (1): 1–8. July 1988. doi:10.1038/clpt.1988.104. PMID 3390996.
- ↑ "Cocaine use and risk of stroke: a systematic review". Drug and Alcohol Dependence 142: 1–13. September 2014. doi:10.1016/j.drugalcdep.2014.06.041. PMID 25066468.
- ↑ 28.0 28.1 "Cocaine: history, social implications, and toxicity--a review". Disease-a-Month 55 (1): 6–38. January 2009. doi:10.1016/j.disamonth.2008.10.002. PMID 19081448.
- ↑ "Fentanyl-Adulterated Cocaine: Strategies To Address The New Normal". 25 April 2019. https://www.addictionpolicy.org/post/fentanyl-adulterated-cocaine-strategies-to-address-the-new-normal.
- ↑ "Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017". Lancet 392 (10159): 1736–1788. November 2018. doi:10.1016/S0140-6736(18)32203-7. PMID 30496103.
- ↑ "Ancient Use of Coca Leaves in the Peruvian Central Highlands". Journal of Anthropological Research 71 (2): 231–258. June 2015. doi:10.3998/jar.0521004.0071.204.
- ↑ "The role of coca in the history, religion, and medicine of South American Indians". Economic Botany 24 (4): 422–438. October 1970. doi:10.1007/BF02860746.
- ↑ "Travelling to new heights: practical high altitude medicine". British Journal of Hospital Medicine 69 (6): 348–352. June 2008. doi:10.12968/hmed.2008.69.6.29626. PMID 18646420.
- ↑ World Drug Report 2021: Booklet 4. [S.l.]: United Nations Office on Drugs and Crime. 2021. p. 35. ISBN 978-92-1-148361-1. https://www.unodc.org/res/wdr2021/field/WDR21_Booklet_4.pdf.
- ↑ "How well do international drug conventions protect public health?". Lancet 379 (9810): 84–91. January 2012. doi:10.1016/s0140-6736(11)61423-2. PMID 22225673. "The international treaties have also constrained national policy experimentation because they require nation states to criminalise drug use".
- ↑ "Drug Fact Sheet: Cocaine". Drug Enforcement Agency. https://www.dea.gov/sites/default/files/2020-06/Cocaine-2020_1.pdf.
- ↑ Berkowitz, Aaron L. (2022). "Chapter 10: Pupillary Control & Approach to Anisocoria: Cranial Nerves 2 & 3" (in English). Clinical Neurology & Neuroanatomy: A Localization-Based Approach (Digital) (2nd ed.). McGraw Hill. ISBN 978-1260453362.
- ↑ "Is cocaine a safe topical agent for use during endoscopic sinus surgery?". The Laryngoscope 126 (8): 1721–1723. August 2016. doi:10.1002/lary.25836. PMID 27075241.
- ↑ "Does cocaine still have a role in nasal surgery?". Drug Safety 20 (1): 9–13. January 1999. doi:10.2165/00002018-199920010-00002. PMID 9935273.
- ↑ "'Moffett's' solution: a review of the evidence and scientific basis for the topical preparation of the nose". Clinical Otolaryngology and Allied Sciences 29 (6): 582–587. December 2004. doi:10.1111/j.1365-2273.2004.00894.x. PMID 15533141.
- ↑ "Drug Approval Package: Goprelto (cocaine hydrochloride)". 30 April 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209963Orig1s000TOC.cfm.
- ↑
 One or more of the preceding sentences incorporates text from a work now in the public domain: "Numbrino: FDA-Approved Drugs". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=209575.
One or more of the preceding sentences incorporates text from a work now in the public domain: "Numbrino: FDA-Approved Drugs". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=209575.
- ↑ 43.0 43.1 World Health Organization (2004). Neuroscience of psychoactive substance use and dependence. World Health Organization. p. 89. ISBN 978-9241562355. https://books.google.com/books?id=G9OhG-dZdAwC&pg=PA89.
- ↑ World Health Organization (2007). International medical guide for ships. World Health Organization. p. 242. ISBN 978-9241547208. https://books.google.com/books?id=ptVjyRs7AdsC&pg=PA242.
- ↑ 45.0 45.1 "Cocaine use and risk of stroke: a systematic review". Drug and Alcohol Dependence 142: 1–13. September 2014. doi:10.1016/j.drugalcdep.2014.06.041. PMID 25066468.
- ↑ "Substance Use, Intoxication, and Withdrawal in the Critical Care Setting". Critical Care Clinics 33 (3): 543–558. July 2017. doi:10.1016/j.ccc.2017.03.003. PMID 28601134.
- ↑ Personality Traits and Drug Consumption. A Story Told by Data. Springer, Cham. 2019. doi:10.1007/978-3-030-10442-9. ISBN 978-3-030-10441-2.
- ↑ "The tradition of chewing coca". https://www.pri.org/stories/2011-04-01/tradition-chewing-coca.
- ↑ 49.0 49.1 "Wilderness Medical Society consensus guidelines for the prevention and treatment of acute altitude illness". Wilderness & Environmental Medicine 21 (2): 146–155. June 2010. doi:10.1016/j.wem.2010.03.002. PMID 20591379. https://oyyum.net/Wilderness/pdf/wilderness-medical.pdf. (mirror: [1])
- ↑ "Cocaine in herbal tea". JAMA 255 (1): 40. January 1986. doi:10.1001/jama.1986.03370010042021. PMID 3940302.
- ↑ "DrugFacts: Cocaine". National Institute on Drug Abuse. April 2013. https://www.drugabuse.gov/publications/drugfacts/cocaine.
- ↑ "The Dangers Of Snorting Cocaine (Insufflation)" (in en-US). https://vertavahealth.com/cocaine/insufflation/.
- ↑ 53.0 53.1 53.2 "Effects of route of administration on cocaine-induced dopamine transporter blockade in the human brain". Life Sciences 67 (12): 1507–1515. August 2000. doi:10.1016/S0024-3205(00)00731-1. PMID 10983846.
- ↑ "Sniffing Around the History of the McDonald's 'Cocaine Spoon'" (in en). 19 March 2021. https://www.mentalfloss.com/article/642413/mcdonalds-cocaine-spoon-controversy.
- ↑ "Cocaine terminology". https://www.cesar.umd.edu/cesar/drugs/cocaine.asp#Terminology.
- ↑ "Hepatitis C: a review and update". Journal of the American Academy of Dermatology 44 (2): 159–182. February 2001. doi:10.1067/mjd.2001.109311. PMID 11174373.
- ↑ "An automated assay of the behavioral effects of cocaine injections in adult Drosophila". Journal of Neuroscience Methods 137 (2): 181–184. August 2004. doi:10.1016/j.jneumeth.2004.02.023. PMID 15262059.
- ↑ "Appendix B: Production of Cocaine Hydrochloride and Cocaine Base". US Justice Dep. https://www.justice.gov/oig/special/9712/appb.htm.
- ↑ "Anhydroecgonine methyl ester, a cocaine pyrolysis product, may contribute to cocaine behavioral sensitization". Toxicology 376: 44–50. February 2017. doi:10.1016/j.tox.2016.04.009. PMID 27129946.
- ↑ "A rose by another name: crack pipe". Lincoln Journal Star. 16 January 2005. https://www.journalstar.com/news/local/article_28e66c86-1ef8-52dc-ac0a-f3933ed6ec5a.html.
- ↑ "Cocaine". https://www.talktofrank.com/drug/cocaine.
- ↑ "Drug harms in the UK: a multicriteria decision analysis". Lancet 376 (9752): 1558–65. November 2010. doi:10.1016/S0140-6736(10)61462-6. PMID 21036393.
- ↑ 63.0 63.1 "Overdose Death Rates". By National Institute on Drug Abuse (NIDA). https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates.
- ↑ "Development of a rational scale to assess the harm of drugs of potential misuse". Lancet 369 (9566): 1047–53. March 2007. doi:10.1016/S0140-6736(07)60464-4. PMID 17382831.
- ↑ "Effects of cocaine on cortisol secretion in humans". The American Journal of the Medical Sciences 310 (2): 61–4. August 1995. doi:10.1097/00000441-199508000-00004. PMID 7631644.
- ↑ "Tolerance to cocaine in brain stimulation reward following continuous cocaine infusions". Pharmacology, Biochemistry, and Behavior 122: 246–52. July 2014. doi:10.1016/j.pbb.2014.04.006. PMID 24768900.
- ↑ "On-body Sensing of Cocaine Craving, Euphoria and Drug-Seeking Behavior Using Cardiac and Respiratory Signals". Proceedings of the ACM on Interactive, Mobile, Wearable and Ubiquitous Technologies 3 (2): 1–31. 21 June 2019. doi:10.1145/3328917.
- ↑ "Extended access of cocaine self-administration results in tolerance to the dopamine-elevating and locomotor-stimulating effects of cocaine". Journal of Neurochemistry 128 (2): 224–32. January 2014. doi:10.1111/jnc.12452. PMID 24102293.
- ↑ 69.0 69.1 Mechanisms Mediating Sex Differences in the Effects of Cocaine. University of Michigan. 2008. p. 3. ISBN 978-0-549-99458-9. https://books.google.com/books?id=AF8zjRBtSuIC&pg=PA3. Retrieved 25 September 2012.
- ↑ "Substance abuse and violence: A review of the literature". Aggression and Violent Behavior 8 (2): 155–174. March–April 2003. doi:10.1016/S1359-1789(01)00057-X.
- ↑ "Cocaine and Cardiotoxicity: A Literature Review". Cureus 13 (4): e14594. April 2021. doi:10.7759/cureus.14594. ISSN 2168-8184. PMID 34036012.
- ↑ "Smoke Gets in the Eye: A systematic review of case reports of ocular complications of crack cocaine use". Drug and Alcohol Review 41 (2): 347–355. August 2021. doi:10.1111/dar.13366. PMID 34337815.
- ↑ "The long-term effects of cocaine use on cognitive functioning: A systematic critical review". Behavioural Brain Research 348: 241–262. August 2018. doi:10.1016/j.bbr.2018.04.005. PMID 29673580.
- ↑ Biological Psychiatry. 2 (2 ed.). Wiley. 2002. p. 528. ISBN 978-0-471-49198-9.
- ↑ "Cocaine abusers do not show loss of dopamine transporters with age". Life Sciences 61 (11): 1059–65. 8 August 1997. doi:10.1016/s0024-3205(97)00614-0. PMID 9307051.
- ↑ "Decreased brain dopamine cell numbers in human cocaine users". Psychiatry Research 168 (3): 173–80. August 2009. doi:10.1016/j.psychres.2008.10.034. PMID 19233481.
- ↑ "Cocaine-induced breakdown of the blood–brain barrier and neurotoxicity". International Review of Neurobiology 88: 297–334. 2009. doi:10.1016/S0074-7742(09)88011-2. ISBN 978-0-12-374504-0. PMID 19897082.
- ↑ Karch's pathology of drug abuse (4 ed.). Boca Raton: CRC Press. 2009. p. 70. ISBN 978-0-8493-7881-2. https://books.google.com/books?id=G9E7gfJq0KkC&pg=PA70.
- ↑ "Physical complications of substance abuse: what the psychiatrist needs to know". Curr Opin Psychiatry 16 (3): 291–296. 2003. doi:10.1097/00001504-200305000-00004.
- ↑ Pagliaros' Comprehensive Guide to Drugs and Substances of Abuse. Washington, D.C.: American Pharmacists Association. 2004. ISBN 978-1-58212-066-9. https://archive.org/details/pagliaroscompreh0000pagl.
- ↑ "Levamisole: a dangerous new cocaine adulterant". Clinical Pharmacology and Therapeutics 88 (3): 408–11. September 2010. doi:10.1038/clpt.2010.156. PMID 20668440.
- ↑ "Levamisole and cocaine synergism: a prevalent adulterant enhances cocaine's action in vivo". Neuropharmacology 79: 590–5. April 2014. doi:10.1016/j.neuropharm.2014.01.002. PMID 24440755.
- ↑ "Cocaine/levamisole-associated autoimmune syndrome: a disease of neutrophil-mediated autoimmunity". Current Opinion in Hematology 25 (1): 29–36. January 2018. doi:10.1097/MOH.0000000000000393. PMID 29211697.
- ↑ "The Cardiovascular Effects of Cocaine". Journal of the American College of Cardiology 70 (1): 101–113. July 2017. doi:10.1016/j.jacc.2017.05.014. PMID 28662796.
- ↑ "Molecular genetics of cocaine use disorders in humans". Molecular Psychiatry 27 (1): 624–639. January 2022. doi:10.1038/s41380-021-01256-1. PMID 34453125.
- ↑ "Cocaine and the AP-1 transcription factor complex". Annals of the New York Academy of Sciences 844 (1): 1–6. May 1998. doi:10.1111/j.1749-6632.1998.tb08216.x. PMID 9668659. Bibcode: 1998NYASA.844....1H. https://zenodo.org/record/1230756.
- ↑ 88.0 88.1 "Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine". Nature 401 (6750): 272–6. September 1999. doi:10.1038/45790. PMID 10499584. Bibcode: 1999Natur.401..272K.
- ↑ 89.0 89.1 "Striatal cell type-specific overexpression of DeltaFosB enhances incentive for cocaine". The Journal of Neuroscience 23 (6): 2488–93. March 2003. doi:10.1523/JNEUROSCI.23-06-02488.2003. PMID 12657709.
- ↑ 90.0 90.1 "DeltaFosB: a sustained molecular switch for addiction". Proceedings of the National Academy of Sciences of the United States of America 98 (20): 11042–6. September 2001. doi:10.1073/pnas.191352698. PMID 11572966. Bibcode: 2001PNAS...9811042N.
- ↑ "Cocaine induces DNA damage in distinct brain areas of female rats under different hormonal conditions". Clinical and Experimental Pharmacology & Physiology 41 (4): 265–9. April 2014. doi:10.1111/1440-1681.12218. PMID 24552452.
- ↑ "Single exposure to cocaine or ecstasy induces DNA damage in brain and other organs of mice". Addiction Biology 15 (1): 96–9. January 2010. doi:10.1111/j.1369-1600.2009.00179.x. PMID 19878142.
- ↑ "Epigenome Maintenance in Response to DNA Damage". Molecular Cell 62 (5): 712–27. June 2016. doi:10.1016/j.molcel.2016.04.006. PMID 27259203.
- ↑ "[Substance Abuse and White Matter: Findings, Limitations, and Future of Diffusion Tensor Imaging Research"] (in en). Drug and Alcohol Dependence 197 (4): 288–298. 2019. doi:10.1016/j.drugalcdep.2019.02.005. PMID 30875650.
- ↑ "Cocaine dependence". Annual Review of Medicine 40: 149–61. 1989. doi:10.1146/annurev.me.40.020189.001053. PMID 2658744.
- ↑ "The Epidemic That Wasn't". The New York Times. 2009-01-27. https://www.nytimes.com/2009/01/27/health/27coca.html.
- ↑ "Growth, development, and behavior in early childhood following prenatal cocaine exposure: a systematic review". JAMA (Jama.ama-assn.org) 285 (12): 1613–1625. March 2001. doi:10.1001/jama.285.12.1613. PMID 11268270.
- ↑ NIDA – Research Report Series – Cocaine Abuse and Addiction
- ↑ "Street Drugs and pregnancy". March of Dimes. http://www.marchofdimes.org/pregnancy/illicit-drug-use-during-pregnancy.aspx.
- ↑ "All-cause and cause-specific mortality among people with regular or problematic cocaine use: a systematic review and meta-analysis". Addiction 116 (4): 725–742. April 2021. doi:10.1111/add.15239. PMID 32857457.
- ↑ "Cocaine pharmacokinetics in humans". Journal of Ethnopharmacology 3 (2–3): 353–66. 1981. doi:10.1016/0378-8741(81)90063-5. PMID 7242115.; Jones, supra note 19; Wilkinson et al., Van Dyke et al.
- ↑ "The mystery of the human proton-organic cation antiporter: One transport protein or many?". Pharmacology & Therapeutics 239: 108283. September 2022. doi:10.1016/j.pharmthera.2022.108283. PMID 36162727.
- ↑ "Urinary excretion of cocaine, benzoylecgonine, and ecgonine methyl ester in humans". Journal of Analytical Toxicology 12 (6): 301–6. November 1988. doi:10.1093/jat/12.6.301. PMID 3244269.
- ↑ "Major and minor metabolites of cocaine in human plasma following controlled subcutaneous cocaine administration". Journal of Analytical Toxicology 30 (8): 501–10. October 2006. doi:10.1093/jat/30.8.501. PMID 17132243. https://openurl.ingenta.com/content/nlm?genre=article&issn=0146-4760&volume=30&issue=8&spage=501&aulast=Kolbrich.
- ↑ "Cocaine Metabolites in Hair". 30 December 2015. https://www.chemistryviews.org/details/news/8693061/Cocaine_Metabolites_in_Hair.html.
- ↑ "Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin". Synapse 39 (1): 32–41. January 2001. doi:10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. PMID 11071707. (Table V. on page 37)
- ↑ "D1 dopamine receptor: a putative neurochemical and behavioral link to cocaine action". Journal of Cellular Physiology 191 (1): 17–27. April 2002. doi:10.1002/jcp.10078. PMID 11920678.
- ↑ "Cocaine inhibits 5-HT3 receptor function in neurons from transgenic mice overexpressing the receptor". European Journal of Pharmacology 459 (2–3): 167–9. January 2003. doi:10.1016/S0014-2999(02)02867-4. PMID 12524142.
- ↑ "Contribution of serotonin (5-hydroxytryptamine; 5-HT) 5-HT2 receptor subtypes to the hyperlocomotor effects of cocaine: acute and chronic pharmacological analyses". The Journal of Pharmacology and Experimental Therapeutics 310 (3): 1246–54. September 2004. doi:10.1124/jpet.104.068841. PMID 15131246.
- ↑ "The binding sites for cocaine and dopamine in the dopamine transporter overlap". Nature Neuroscience 11 (7): 780–9. July 2008. doi:10.1038/nn.2146. PMID 18568020.
- ↑ "Sigma Receptors Play Role in Cocaine-induced Suppression of Immune System". Sciencedaily.com. 6 May 2003. https://www.sciencedaily.com/releases/2003/05/030506073758.htm.
- ↑ "Role of dopamine and glutamate receptors in cocaine-induced social effects in isolated and grouped male OF1 mice". Pharmacology Biochemistry and Behavior 82 (3): 478–87. November 2005. doi:10.1016/j.pbb.2005.10.003. PMID 16313950.
- ↑ "Cardiovascular disorders associated with cocaine use: myths and truths". Pharmacology & Therapeutics 97 (3): 181–222. March 2003. doi:10.1016/S0163-7258(02)00329-7. PMID 12576134.
- ↑ "Drugbank website "drug card", "(DB00907)" for Cocaine: Giving ten targets of the molecule in vivo, including dopamine/serotonin sodium channel affinity & K-opioid affinity". Drugbank.ca. https://www.drugbank.ca/drugs/DB00907.
- ↑ "The pineal gland is critical for circadian Period1 expression in the striatum and for circadian cocaine sensitization in mice". Neuropsychopharmacology 28 (12): 2117–23. December 2003. doi:10.1038/sj.npp.1300254. PMID 12865893.
- ↑ "Regulation of dopaminergic transmission and cocaine reward by the Clock gene". Proceedings of the National Academy of Sciences of the United States of America 102 (26): 9377–81. June 2005. doi:10.1073/pnas.0503584102. PMID 15967985. Bibcode: 2005PNAS..102.9377M.
- ↑ "Cocaine Blocks Effects of Hunger Hormone, Ghrelin, Via Interaction with Neuronal Sigma-1 Receptors". Molecular Neurobiology 56 (2): 1196–1210. February 2019. doi:10.1007/s12035-018-1140-7. PMID 29876881.
- ↑ "Acute effects of intravenous cocaine administration on serum concentrations of ghrelin, amylin, glucagon-like peptide-1, insulin, leptin and peptide YY and relationships with cardiorespiratory and subjective responses". Drug and Alcohol Dependence 180: 68–75. November 2017. doi:10.1016/j.drugalcdep.2017.07.033. PMID 28881319.
- ↑ "Cocaine effects on behavioral responding to a novel object placed in a familiar environment". Pharmacology Biochemistry and Behavior 88 (3): 265–71. January 2008. doi:10.1016/j.pbb.2007.08.010. PMID 17897705.
- ↑ Modern Medical Toxicology (4th ed.), Jaypee, 2013, pp. 553–554, ISBN 978-93-5025-965-8
- ↑ "DEA fact sheet". https://www.dea.gov/factsheets/cocaine.
- ↑ "Content Background: Chemical Characteristics of Cocaine". https://www.chm.bris.ac.uk/motm/cocaine/cocaineh.htm.
- ↑ "Pharmacokinetics and pharmacodynamics of methylecgonidine, a crack cocaine pyrolyzate". The Journal of Pharmacology and Experimental Therapeutics 307 (3): 1179–87. December 2003. doi:10.1124/jpet.103.055434. PMID 14561847.
- ↑ "Evidence for cocaine and methylecgonidine stimulation of M(2) muscarinic receptors in cultured human embryonic lung cells". British Journal of Pharmacology 132 (2): 451–60. January 2001. doi:10.1038/sj.bjp.0703819. PMID 11159694.
- ↑ "Studies on hydrolytic and oxidative metabolic pathways of anhydroecgonine methyl ester (methylecgonidine) using microsomal preparations from rat organs". Chemical Research in Toxicology 15 (12): 1543–8. December 2002. doi:10.1021/tx0255828. PMID 12482236.
- ↑ "Crack – A Cheap and Deadly Cocaine Is a Spreading Menace.". Time. 2 June 1986. pp. 16–18. https://www.ojp.gov/ncjrs/virtual-library/abstracts/crack-cheap-and-deadly-cocaine-spreading-menace.
- ↑ Principles of addiction medicine. Lippincott Williams & Wilkins. 2009. p. 137. ISBN 978-0-7817-7477-2. https://books.google.com/books?id=j6GGBud8DXcC&pg=PT166. Retrieved 5 January 2014.
- ↑ Hip Hop America. Viking Penguin. 1998. p. 40. https://books.google.com/books?id=8nw6VlsqOUsC&pg=PA40.
- ↑ "It's legal to manufacture cocaine and heroin for medical use — and Britain is the world's biggest exporter". https://www.businessinsider.com/britain-is-the-worlds-biggest-exporter-of-legal-cocaine-and-heroin-2018-4.
- ↑ "The Coca Story Goes Way Beyond Cola.". Taste. 26 October 2021. https://tastecooking.com/the-coca-story-goes-way-beyond-the-cola/.
- ↑ "Cocaine: Effects, Hazards & Warnings". https://www.drugs.com/illicit/cocaine.html.
- ↑ "The Standard Low Dose of Oral Cocaine: Used for Treatment of Cocaine Dependence.". Substance Abuse 15 (4): 215–220. 1994. http://www.dronet.org/sostanze/sos_pdf/cocaine21.pdf.
- ↑ "Identification and quantitation of alkaloids in coca tea". Forensic Science International 77 (3): 179–189. February 1996. doi:10.1016/0379-0738(95)01860-3. PMID 8819993.
- ↑ 134.0 134.1 "Tropane alkaloid biosynthesis. A century-old problem unresolved". Natural Product Reports 18 (5): 494–502. October 2001. doi:10.1039/b001713m. PMID 11699882.
- ↑ Medicinal Natural Products. Chichester: Wiley-Blackwell. 2009. ISBN 978-0-470-74276-1.
- ↑ "The Biosynthesis of Tropane Alkaloids in Datura stramonium: The Identity of the Intermediates between N-Methylpyrrolinium Salt and Tropinone". J. Am. Chem. Soc. 119 (45): 10929–10934. 1997. doi:10.1021/ja964461p.
- ↑ "N-methylputrescine oxidation during cocaine biosynthesis: study of prochiral methylene hydrogen discrimination using the remote isotope method". Organic Letters 2 (1): 3–5. January 2000. doi:10.1021/ol990940s. PMID 10814231.
- ↑ "Late intermediates in the biosynthesis of cocaine: 4-(1-methyl-2-pyrrolidinyl)-3-oxobutanoate and methyl ecgonine". J. Am. Chem. Soc. 113 (24): 9286–9292. 1991. doi:10.1021/ja00024a039.
- ↑ "The biosynthesis of the benzoyl moiety of cocaine". Phytochemistry 27 (8): 2553–2556. 1988. doi:10.1016/0031-9422(88)87026-2. Bibcode: 1988PChem..27.2553L.
- ↑ "Biogenesis of hyoscyamine". Nature 174 (4431): 650–1. October 1954. doi:10.1038/174650a0. PMID 13203600. Bibcode: 1954Natur.174..650L.
- ↑ "Strategies for the genetic manipulation of alkaloid-producing pathways in plants". Planta Medica 57 (7 Suppl): S27-35. October 1991. doi:10.1055/s-2006-960226. PMID 17226220.
- ↑ "Tropane and Related Alkaloids". Top. Curr. Chem.. Topics in Current Chemistry 209: 175. 2000. doi:10.1007/3-540-48146-X. ISBN 978-3-540-66573-1.
- ↑ "Two tropinone reducing enzymes from Datura stramonium transformed root cultures". Phytochemistry 31 (4): 1135–1138. 1992. doi:10.1016/0031-9422(92)80247-C. Bibcode: 1992PChem..31.1135P.
- ↑ "Specificities of the enzymes of N-alkyltropane biosynthesis in Brugmansia and Datura". Phytochemistry 52 (5): 871–8. November 1999. doi:10.1016/S0031-9422(99)00293-9. PMID 10626376. Bibcode: 1999PChem..52..871B.
- ↑ "Genetically modified tobacco plant produces cocaine in its leaves". https://www.newscientist.com/article/2348568-genetically-modified-tobacco-plant-produces-cocaine-in-its-leaves/.
- ↑ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 9th edition, Biomedical Publications, Seal Beach, California, 2011, pp. 390–394.
- ↑ "Meet the Chemist Behind Many Popular—and Faulty—Police Drug Kits". Pacific Standard. 22 June 2016. https://psmag.com/news/meet-the-chemist-behind-many-popular-and-faulty-police-drug-kits.
- ↑ "How a $2 Roadside Drug Test Sends Innocent People to Jail". The New York Times. 7 July 2016. https://www.nytimes.com/2016/07/10/magazine/how-a-2-roadside-drug-test-sends-innocent-people-to-jail.html.
- ↑ "Semiquantitative screening test for cocaine". Analytical Chemistry 55 (4): 795–796. 1 April 1983. doi:10.1021/ac00255a048. ISSN 0003-2700.
- ↑ "Annual prevalence of use of drugs, by region and globally, 2016". World Drug Report 2018. United Nations Office on Drugs and Crime. 2018. https://dataunodc.un.org/drugs/prevalence_regional. Retrieved 7 July 2018.
- ↑ 151.0 151.1 "World Drug Report 2016 (interactive map)". United Nations Office on Drugs and Crime. 2016. https://www.unodc.org/wdr2016/interactive-map.html.
- ↑ "Statistical Bulletin 2018 — prevalence of drug use". https://www.emcdda.europa.eu/data/stats2018/gps_en.
- ↑ The State of the Drugs Problem in Europe 2008. Luxembourg: European Monitoring Centre for Drugs and Drug Addiction. 2008. pp. 58–62. https://www.emcdda.europa.eu/attachements.cfm/att_64227_EN_EMCDDA_AR08_en.pdf. Retrieved 31 December 2013.
- ↑ "Cocaine in sewage: London tops league table". BBC News. 4 June 2015. https://www.bbc.co.uk/news/uk-33009682.
- ↑ "Cocaine & Crack". Erowid.org. https://www.erowid.org/chemicals/cocaine/cocaine.shtml.
- ↑ 156.0 156.1 "Field Listing – Illicit drugs (by country)". Cia.gov. https://www.cia.gov/library/publications/the-world-factbook/fields/2086.html.
- ↑ Alkaloids: Nature's Curse or Blessing?. Weinheim: Wiley-VCH. 2002. p. 304. ISBN 978-3-906390-24-6.
- ↑ "Cocaine's use in ophthalmology: our 100-year heritage". Survey of Ophthalmology 29 (4): 300–6. 1985. doi:10.1016/0039-6257(85)90153-5. PMID 3885453.
- ↑ "Cocaine: history, epidemiology, human pharmacology, and treatment. a perspective on a new debut for an old girl". Clinical Toxicology 8 (2): 149–78. 1975. doi:10.3109/15563657508988061. PMID 1097168.
- ↑ "Drug that spans the ages: The history of cocaine". London: The Independent (UK). 2006. https://www.independent.co.uk/news/uk/this-britain/drug-that-spans-the-ages-the-history-of-cocaine-468286.html.
- ↑ Joyfull Newes out of the Newe Founde Worlde. New York: Alfred Knopf. 1925.
- ↑ "InterAndean Institute of Coca Sciences". https://www.cienciadelacoca.org/CocaVega.html.
- ↑ "Ueber das Erythroxylin, dargestellt aus den Blättern des in Südamerika cultivirten Strauches Erythroxylon Coca". Archiv der Pharmazie 132 (2): 141–150. 1855. doi:10.1002/ardp.18551320208. https://zenodo.org/record/1424529.
- ↑ 164.0 164.1 "Ueber eine neue organische Base in den Cocablättern". Archiv der Pharmazie 153 (2 and 3): 129–155; 291–308. 1860. doi:10.1002/ardp.18601530202. https://zenodo.org/record/1424541.
- ↑ Harper, Douglas. "Cocaine". Online Etymology Dictionary. https://www.etymonline.com/?term=Cocaine.
- ↑ "Chemistry, design, and structure-activity relationship of cocaine antagonists". Chemical Reviews 100 (3): 925–1024. March 2000. doi:10.1021/cr9700538. PMID 11749256. https://www.erowid.org/archive/rhodium/pdf/cocaineanalogs.pdf. "Page 970 (46th page of article) first, ninth, and tenth lines".
- ↑ (a) "Synthese der Ecgoninsäure" (in de). Liebigs Ann. 326 (1–2): 23. 1903. doi:10.1002/jlac.19033260105. (b) "LXIII. A synthesis of tropinone". J. Chem. Soc., Trans. 111: 762–768. 1917. doi:10.1039/CT9171100762. https://zenodo.org/record/1429739. (c) "Die Synthese des Tropinons, Pseudopelletierins, Lobelanins und verwandter Alkaloide unter physiologischen Bedingungen" (in de). Liebigs Ann. 518: 1–37. 1935. doi:10.1002/jlac.19355180102.
- ↑ "Vassily von Anrep, forgotten pioneer of regional anesthesia". Anesthesiology 90 (3): 890–5. March 1999. doi:10.1097/00000542-199903000-00033. PMID 10078692.
- ↑ "Practical comments on the use and abuse of cocaine". New York Medical Journal 42: 294–295. 1885.
- ↑ "An experimental study". New York Medical Journal 42: 483. 1885.
- ↑ "Experience Vin Mariani today | Grupo Mariani S.A". Cocanaturally.com. https://www.cocanaturally.com/.
- ↑ Cocaine Papers. Stonehill. 1975. ISBN 0-88373-010-3.
- ↑ "How a Young Sigmund Freud Researched & Got Addicted to Cocaine, the New "Miracle Drug," in 1894". https://www.openculture.com/2014/04/igmund-freud-researched-got-addicted-to-cocaine.html.
- ↑ "Sigmund Freud and Cocaine". https://cocaine.org/cokespoon.htm.
- ↑ "America's First Cocaine Epidemic". The Wilson Quarterly 13 (3): 59–64. 1989. PMID 11619697. https://www.jstor.org/stable/40257908.
- ↑ Looking Up At Down: The Emergence of Blues Culture. Philadelphia: Temple University Press. 1989. p. 207. ISBN 0-87722-583-4. https://archive.org/details/lookingupatdowne0000barl.
- ↑ Cocaine: An Unauthorized Biography. Picador. 2003. ISBN 978-0-312-42226-4.
- ↑ "Catchin' Cab: The Magic of Calloway". Billboard: 3. August 14, 1993. https://books.google.com/books?id=6BEEAAAAMBAJ&q=billboard+minnie+the+moocher+1931&pg=PA3.
- ↑ (in en) The Big Broadcast (1932) (Full Movie), pp. at 1:20:29, https://www.youtube.com/watch?v=nCC4zlrC5rQ&t=1h20m30s, retrieved 2022-01-13
- ↑ "Jeevan Vasagar: cocaine-based "wonder drug" tested on concentration camp inmates". Amphetamines.com. 19 November 2002. https://amphetamines.com/nazidrug.html.
- ↑ 181.0 181.1 "Cocaine: Hidden in Plain Sight". The New York Times. 10 June 2007. https://www.nytimes.com/2007/06/10/fashion/10cocaine.html.
- ↑ "The Buyers – A Social History of America's Most Popular Drugs". FRONTLINE. PBS. https://www.pbs.org/wgbh/pages/frontline/shows/drugs/buyers/socialhistory.html.
- ↑ Treatment of Black Alcoholics. Psychology Press. 1985. ISBN 978-0-86656-403-8. https://books.google.com/books?id=DA7SmDh-X5cC&pg=PA167. Retrieved 18 May 2017.
- ↑ Cocaine Changes: The Experience of Using and Quitting. Temple University Press. June 1992. p. 95. ISBN 978-1-56639-013-2. https://archive.org/details/cocainechangesex00wald. Retrieved 18 May 2017.
- ↑ 185.0 185.1 "Cocaine". Pharmacology for Anesthetists. p. 27. https://books.google.com/books?id=_Du2bfrO9FwC&pg=PA27.
- ↑ "Apple Sanity – Fetish – Blow: War on Drugs VS. Cocaine". Applesanity.com. 17 June 2008. https://www.applesanity.com/fetish/blow/.
- ↑ "Cocaine Market". Havocscope.com. 28 April 2008. https://www.havocscope.com/tag/cocaine/.
- ↑ WHO/UNICRI (4 February 2010). "The WHO Cocaine Project". Transnational Institute. https://www.tni.org/article/who-cocaine-project.
- ↑ "Cocaine use doubles in a decade". Sydney Morning Herald. 15 October 2010. https://www.smh.com.au/lifestyle/wellbeing/cocaine-use-doubles-in-a-decade-20101015-16mli.html.
- ↑ EMCDDA (2007). "EMCDDA Retail Cocaine Purity Study". https://www.emcdda.europa.eu/stats09/ppptab7a.
- ↑ "The world's first cocaine bar" (in en-GB). The Guardian. 18 August 2009. ISSN 0261-3077. https://www.theguardian.com/world/2009/aug/19/bolivia-cocaine-bar-route-36.
- ↑ "The Cato Accord: Bolivia's Humane and Effective Approach to Controlling Coca Cultivation". https://ain-bolivia.org/wp-content/uploads/The-Cato-Accord-Bolivias-Humane-and-Effective-Approach-to-Controlling-Coca-Cultivation.pdf.
- ↑ "Therapeutic Goods (Poisons Standard—October 2023) Instrument 2023". Australian Government. 26 September 2023. https://www.legislation.gov.au/F2023L01294/asmade/text.
- ↑ "Illicit drug use". 13 December 2023. https://www.aihw.gov.au/reports/illicit-use-of-drugs/illicit-drug-use.
- ↑ "Misuse of Drugs Act 1981". Government of Western Australia Department of Justice Parliamentary Counsel's Office. https://www.legislation.wa.gov.au/legislation/prod/filestore.nsf/FileURL/mrdoc_46172.pdf/$FILE/Misuse%20Of%20Drugs%20Act%201981%20-%20%5B08-f0-00%5D.pdf?OpenElement.
- ↑ Gootenberg 1999, p. 37.
- ↑ Madge 2001, p. 106.
- ↑ "Narcotic drug addiction" (in en). American Medicine (American-Medicine Publishing Company): 799. November 1915. https://books.google.com/books?id=tvAAAAAAYAAJ&pg=PA799. Retrieved 29 April 2018.
- ↑ 199.0 199.1 199.2 Gootenberg 1999, p. 40.
- ↑ Race and Crime: Geographies of Injustice. Oakland, California: University of California Press. 2018. pp. 207–209. ISBN 978-0-520-29418-9. https://books.google.com/books?id=2ChtDwAAQBAJ. Retrieved 21 November 2021.
- ↑ The Political Roots of Racial Tracking in American Criminal Justice. New York, NY: Cambridge University Press. 2015. p. 270. ISBN 978-1-107-02297-3. https://books.google.com/books?id=QKwPBgAAQBAJ&pg=PA270. Retrieved 21 November 2021.
- ↑ Suspect Race: Causes and Consequences of Racial Profiling. New York, NY: Oxford University Press. 2015. p. 7. ISBN 978-0-19-537040-9. https://books.google.com/books?id=3GjDBAAAQBAJ&pg=PA7. Retrieved 21 November 2021.
- ↑ "Cocaine: Seizures, 1998–2003". World Drug Report 2006. 2. New York: United Nations. 2006. https://www.unodc.org/pdf/WDR_2006/wdr2006_chap4_cocaine.pdf.
- ↑ Colombia . CIA World Factbook
- ↑ "Peru Overtakes Colombia as Top Cocaine Producer". NBC News. 31 July 2012. https://worldnews.nbcnews.com/_news/2012/07/31/13045253-us-peru-overtakes-colombia-as-top-cocaine-producer.
- ↑ Cocaine: An Unauthorized Biography. Macmillan. 2003. ISBN 978-0-312-42226-4. https://books.google.com/books?id=9ceLzaeHsZAC&pg=PA462. Retrieved 5 January 2014.
- ↑ "National Drug Threat Assessment 2006". 2006. https://www.justice.gov/ndic/pubs11/18862/index.htm.
- ↑ "Cocaine Seized Worldwide Highest Ever in 2011". Flare Network (Flarenetwork.org). 18 January 2012. https://www.flarenetwork.org/report/top_news_100615/article/cocaine_seized_worldwide_highest_ever_in_2011.htm.
- ↑ "Colombia". State.gov. https://2009-2017.state.gov/r/pa/ei/bgn/35754.htm.
- ↑ "How Much Is a Gram of Coke?". 3 September 2015. https://www.newhealthadvisor.com/How-Much-Is-a-Gram-of-Coke.html.
- ↑ Illegal drugs: America's anguish (2005 ed.). Farmington Hills, Michigan: Thomson Gale. 2006. ISBN 978-1-4144-0419-6. https://archive.org/details/illegaldrugsamer00jaco.
- ↑ "2015 National Drug Threat Assessment Summary". United States Department of Justice: Drug Enforcement Administration. October 2015. pp. 1–2. https://www.dea.gov/docs/2015%20NDTA%20Report.pdf. "Mexican TCOs pose the greatest criminal drug threat to the United States; no other group is currently positioned to challenge them. These Mexican poly-drug organizations traffic heroin, methamphetamine, cocaine, and marijuana throughout the United States, using established transportation routes and distribution networks. ... While all of these Mexican TCOs transport wholesale quantities of illicit drugs into the United States, the Sinaloa Cartel appears to be the most active supplier. The Sinaloa Cartel leverages its expansive resources and dominance in Mexico to facilitate the smuggling and transportation of drugs throughout the United States."
- ↑ "Coast Guard hunts drug-running semi-subs". CNN. 20 March 2008. https://edition.cnn.com/2008/CRIME/03/20/drug.subs/index.html.
- ↑ "Meth Info". Methproject.org. https://methproject.org/Meth_Info/education.php.
- ↑ "Drugs of Abuse". City of Denison Iowa. https://www.denisonia.com/policeDept/amphetamines.asp.
- ↑ "Drugs: Pricing Power". The Economist. 28 June 2007. https://www.economist.com/node/9414607. "Prices: USA around $110/g, Israel/Germany/Britain around $46/g, Colombia $2/g, New Zealand recordbreaking $714.30/g."
- ↑ European Monitoring Centre for Drugs and Drug Addiction (2008). Annual report: the state of the drugs problem in Europe. Luxembourg: Office for Official Publications of the European Communities. p. 59. ISBN 978-92-9168-324-6. https://www.emcdda.europa.eu/attachements.cfm/att_64227_EN_EMCDDA_AR08_en.pdf. Retrieved 31 December 2013.
- ↑ The Cocaine Threat: A Hemispheric Perspective. United States Department of Defense. https://www.defenselink.mil/policy/sections/policy_offices/solic/cn/cocaine2.pdf.
- ↑ United Nations (June 2010). World Drug Report 2010. United Nations Publications. p. 77. ISBN 978-92-1-148256-0. https://books.google.com/books?id=HB9PuEhHahQC&pg=PA77.
General bibliography
- Cocaine: Global Histories. London: Routledge. 1999. ISBN 978-0-203-02646-5. https://archive.org/details/cocaineglobalhist00goot.
- White Mischief: A Cultural History of Cocaine. Edinburgh: Mainstream Publishing Company. 2001. ISBN 978-1-84018-405-1.
- Cocaine: From Medical Marvel to Modern Menace in the United States, 1884–1920. Baltimore and London: The Johns Hopkins University Press. 2000. ISBN 978-0-8018-6230-4. https://archive.org/details/cocainefrommedic00spil.
Further reading
- The Candy Machine: How Cocaine Took Over the World. London: Penguin. 2009. ISBN 978-0-14-103446-1.
External links
- "Cocaine". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/cocaine.
- "Cocaine". European Monitoring Centre for Drugs. https://www.emcdda.europa.eu/publications/drug-profiles/cannabis.
 |
![Delphic analysis regarding 20 popular recreational drugs based on expert opinion in the UK. Cocaine was ranked the 2nd in dependence and physical harm and 3rd in social harm.[64]](/wiki/images/thumb/7/73/Rational_harm_assessment_of_drugs_bar_plot.svg/504px-Rational_harm_assessment_of_drugs_bar_plot.svg.png)
