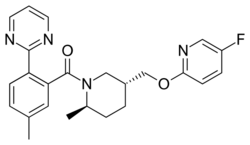
Chemistry:Filorexant
 | |
| Clinical data | |
|---|---|
| Other names | MK-6096; MK6096 |
| Routes of administration | By mouth |
| Drug class | Orexin antagonist |
| ATC code |
|
| Pharmacokinetic data | |
| Elimination half-life | 3–6 hours[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| PDB ligand | |
| Chemical and physical data | |
| Formula | C24H25FN4O2 |
| Molar mass | 420.488 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Filorexant (INN, USAN; developmental code name MK-6096) is an orexin antagonist which was under development by Merck for the treatment of insomnia, depression, diabetic neuropathy, and migraine.[2][3] It is a dual antagonist of the orexin OX1 and OX2 receptors.[4][5] It has a relatively short elimination half-life of 3 to 6 hours.[1] However, it dissociates slowly from the orexin receptors and may thereby have a longer duration.[6] Possibly in relation to this, filorexant shows next-day somnolence similarly to suvorexant.[6] In phase 2 clinical trials, filorexant was found to be effective in the treatment of insomnia,[7] but was not effective in the treatment of major depressive disorder,[8][9][10] painful diabetic neuropathy,[11][12] or migraine.[13] As of May 2015[update], filorexant was no longer listed on Merck's online development pipeline and hence development of the drug appears to have been discontinued.[14][1][2] Development of filorexant may have been discontinued due to lack of differentiation from suvorexant (which was also developed by Merck).[6]
See also
References
- ↑ 1.0 1.1 1.2 "Orexin Receptor Antagonists". Current Sleep Medicine Reports 3 (4): 342–353. 13 November 2017. doi:10.1007/s40675-017-0099-7.
- ↑ 2.0 2.1 "Filorexant". AdisInsight. Springer Nature Switzerland AG. http://adisinsight.springer.com/drugs/800031738.
- ↑ "Orexin in sleep, addiction and more: is the perfect insomnia drug at hand?". Neuropeptides 47 (6): 477–488. December 2013. doi:10.1016/j.npep.2013.10.009. PMID 24215799.
- ↑ "Pharmacological characterization of MK-6096 - a dual orexin receptor antagonist for insomnia". Neuropharmacology 62 (2): 978–987. February 2012. doi:10.1016/j.neuropharm.2011.10.003. PMID 22019562.
- ↑ "Clinical trials update. 2013: year in review". Headache 54 (1): 189–194. January 2014. doi:10.1111/head.12267. PMID 24400767.
- ↑ 6.0 6.1 6.2 "Hypocretins (orexins): The ultimate translational neuropeptides". Journal of Internal Medicine 291 (5): 533–556. May 2022. doi:10.1111/joim.13406. PMID 35043499.
- ↑ "A Phase II Dose-Ranging Study Evaluating the Efficacy and Safety of the Orexin Receptor Antagonist Filorexant (MK-6096) in Patients with Primary Insomnia". The International Journal of Neuropsychopharmacology 19 (8): pyw022. August 2016. doi:10.1093/ijnp/pyw022. PMID 26979830.
- ↑ "Orexin/hypocretin receptor modulation of anxiolytic and antidepressive responses during social stress and decision-making: Potential for therapy". Brain Research 1731: 146085. March 2020. doi:10.1016/j.brainres.2018.12.036. PMID 30590027.
- ↑ "Orexin Receptor Antagonists as Emerging Treatments for Psychiatric Disorders". Neuroscience Bulletin 36 (4): 432–448. April 2020. doi:10.1007/s12264-019-00447-9. PMID 31782044.
- ↑ "Phase II Proof-of-Concept Trial of the Orexin Receptor Antagonist Filorexant (MK-6096) in Patients with Major Depressive Disorder". The International Journal of Neuropsychopharmacology 20 (8): 613–618. August 2017. doi:10.1093/ijnp/pyx033. PMID 28582570.
- ↑ Insomnia and beyond - Exploring the therapeutic potential of orexin receptor antagonists. Frontiers E-books. 11 November 2014. pp. 3–. ISBN 978-2-88919-330-1. https://books.google.com/books?id=meadBQAAQBAJ&pg=PA3.
- ↑ "Orexin Receptor Antagonism in Painful Diabetic Neuropathy: A Phase 2 Trial With Filorexant". The Clinical Journal of Pain 34 (1): 37–43. January 2018. doi:10.1097/AJP.0000000000000503. PMID 28448426.
- ↑ "Randomized controlled trial of the orexin receptor antagonist filorexant for migraine prophylaxis". Cephalalgia 35 (5): 379–388. April 2015. doi:10.1177/0333102414544979. PMID 25106663.
- ↑ "Merck Pipeline". Merck. 2015. http://www.merck.com/research/pipeline/home.html.
External links
 |
