Chemistry:Fluoroacetone
From HandWiki
| |
| Names | |
|---|---|
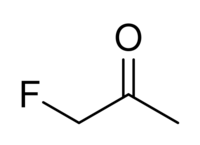
| IUPAC name
1-Fluoropropan-2-one
| |
| Other names
Fluoroacetone; 1-fluoro-2-propanone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C3H5FO | |
| Molar mass | 76.070 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.054 g/mL |
| Boiling point | 75 °C (167 °F; 348 K) |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | DANGER |
| H225, H300, H310, H330 | |
| P210, P233, P240, P241, P242, P243, P260, P262, P264, P270, P271, P280, P284, P301+310, P302+350, P303+361+353, P304+340, P310, P320, P321, P322, P330, P361, P363, P370+378 | |
| Flash point | 7 °C (45 °F; 280 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Fluoroacetone is an organofluorine compound with the chemical formula C3H5FO.[1][2] Under normal conditions, the substance is a colorless liquid. Fluoroacetone is also a highly toxic and flammable compound.[3] Fumes of fluoroacetone can form an explosive mixture with air.
Synthesis
Fluoroacetone can be obtained by a reaction of triethylamine tris-hydrofluoride with bromoacetone.
Applications
Fluoroacetone is used as a catalyst to study the kinetics of the ketone-catalysed decomposition of peroxymonosulfuric acid (Caro's acid).[4] It is also a precursor material for the production of higher fluoroketones.
Fluoroacetone has not been used as a lachrymatory substance in contrast to other halogenated acetone derivatives, such as bromoacetone or chloroacetone.
See also
References
- ↑ "Fluoroacetone Basic information". chemicalbook.com. http://www.chemicalbook.com/ProductChemicalPropertiesCB7853623_EN.htm. Retrieved 1 June 2017.
- ↑ Newallis, Peter E.; Lombardo, Pasquale (1965). "Fluoro Ketones. III. Preparation and Thermal Decomposition of Fluoroacetone Hemiketal Esters" (in English). J. Org. Chem. 30 (11): 3834–3837. doi:10.1021/jo01022a055.
- ↑ "Substance information". echa.europa.eu. https://echa.europa.eu/substance-information/-/substanceinfo/100.006.423. Retrieved 1 June 2017.
- ↑ "Fluoroacetone". sigmaaldrich.com. http://www.sigmaaldrich.com/catalog/product/aldrich/115460?lang=en®ion=RU&gclid=Cj0KEQjw9r7JBRCj37PlltTskaMBEiQAKTzTfCCBAPuOAJJ4w2PZ2SbhBA617Fli51x_CgVDFxLCdd8aAh5w8P8HAQ. Retrieved 1 June 2017.
 |

