Chemistry:Chloroacetone
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-Chloropropan-2-one | |
| Other names
Acetonyl chloride, chloropropanone, 1-chloro-2-propanone, monochloroacetone, 1-chloro-2-ketopropane, 1-chloro-2-oxypropane
UN 1695 | |
| Identifiers | |
3D model (JSmol)
|
|
| 605369 | |
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C3H5ClO | |
| Molar mass | 92.52 g·mol−1 |
| Appearance | Colorless liquid, oxidizes to amber |
| Density | 1.123 g/cm3 |
| Melting point | −44.5 °C (−48.1 °F; 228.7 K) |
| Boiling point | 119 °C (246 °F; 392 K) |
| 10 g/100 mL at 20 °C | |
| Solubility | miscible with alcohol, ether, chloroform |
| Vapor pressure | 1.5 kPa |
| -50.9·10−6 cm3/mol | |
| 2.36 | |
| Hazards | |
| Flash point | 35 °C (95 °F; 308 K) |
| 610 °C (1,130 °F; 883 K) | |
| Explosive limits | 3.4% - ?[1] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
100 mg/kg (rats, oral)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
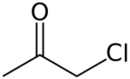
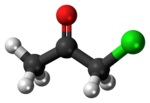
Chloroacetone is a chemical compound with the formula CH
3COCH
2Cl. At STP it is a colourless liquid with a pungent odour.[3] On exposure to light, it turns to a dark yellow-amber colour.[4] It was used as a tear gas in World War I.[5]
Synthesis
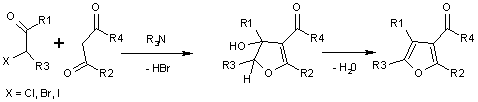
Chloroacetone may be synthesized from the reaction between chlorine and diketene, or by the chlorination of acetone.
Applications
Chloroacetone is used to make dye couplers for colour photography, and is an intermediate in chemical manufacturing.[2] It is also used in the Feist-Benary synthesis of furans.[6]
- Reaction of phenoxide with chloroacetone gives phenoxyacetone,[7] which is used to make a wide variety of different pharmaceuticals. A catalytic amount of potassium iodide is also necessary to facilitate a Finkelstein reaction.
Purification
Chloroacetone purchased from commercial suppliers contains 5% impurities including mesityl oxide, which is not removed by distillation. Mesityl oxide can be oxidized using acidified KMnO4 to form a diol (followed by separation with ether), which is removed on subsequent distillation.[8]
Transportation regulations
Transportation of unstabilized chloroacetone has been banned in the United States by the US Department of Transportation. Stabilized chloroacetone is in hazard class 6.1 (Poison Inhalation Hazard). Its UN number is 1695.
See also
- Bromoacetone
- Dichloroacetone
- Fluoroacetone
- Hexachloroacetone
- Use of poison gas in World War I
References
- ↑ "ICSC:NENG0760 International Chemical Safety Cards (WHO/IPCS/ILO) CDC/NIOSH". Center for Disease Control. 2006-10-11. https://www.cdc.gov/niosh/ipcsneng/neng0760.html.
- ↑ 2.0 2.1 Hathaway, Gloria J.; Proctor, Nick H. (2004). Proctor and Hughes' Chemical Hazards of the Workplace (5 ed.). Wiley-Interscience. pp. 143–144. ISBN 978-0-471-26883-3. https://books.google.com/books?id=y3-Ef3y53PkC&dq=Chloroacetone&pg=PA143. Retrieved 2009-04-16.
- ↑ "Occupational Safety and Health Guideline for Chloroacetone". U.S. Department of Labor - Occupational Safety & Health Administration. http://www.osha.gov/SLTC/healthguidelines/chloroacetone/recognition.html.
- ↑ "CHLOROACETONE". International Programme on Chemical Safety. http://www.inchem.org/documents/icsc/icsc/eics0760.htm.
- ↑ Haber, Ludwig Fritz (1986). The Poisonous Cloud: Chemical Warfare in the First World War. Oxford University Press. ISBN 0-19-858142-4.
- ↑ Li, Jie-Jack; Corey, E. J. (2004). Name Reactions in Heterocyclic Chemistry. Wiley-Interscience. pp. 160. ISBN 978-0-471-30215-5. https://books.google.com/books?id=1N-MZVSesTcC&dq=Chloroacetone&pg=PA160. Retrieved 2009-04-16.
- ↑ Hurd, Charles D.; Perletz, Percy (1946). "Aryloxyacetones1". Journal of the American Chemical Society 68 (1): 38–40. doi:10.1021/ja01205a012. ISSN 0002-7863.
- ↑ Phys. Chem. Chem. Phys., 2000,2, 237-245
External links
 |