Chemistry:Frémy's salt
| |
| |
| Names | |
|---|---|
| IUPAC name
Potassium nitrosodisulfonate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
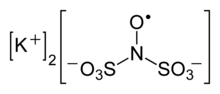
| K2NO(SO3)2 | |
| Molar mass | 268.33 g/mol (potassium salt) |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H260, H302, H312, H332 | |
| P223, P231+232, P280, P301+312, P302+352+312Script error: No such module "Preview warning".Category:GHS errors, P304+340+312Script error: No such module "Preview warning".Category:GHS errors | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Frémy's salt is a chemical compound with the formula (K4[ON(SO3)2]2), sometimes written as (K2[NO(SO3)2]). It a bright yellowish-brown solid, but its aqueous solutions are bright violet.[1][2] The related sodium salt, disodium nitrosodisulfonate (NDS, Na2ON(SO3)2, CAS 29554-37-8) is also referred to as Frémy's salt.[3]
Regardless of the cations, the salts are distinctive because aqueous solutions contain the radical [ON(SO3)2]2−.
Applications
Frémy's salt, being a long-lived free radical, is used as a standard in electron paramagnetic resonance (EPR) spectroscopy, e.g. for quantitation of radicals. Its intense EPR spectrum is dominated by three lines of equal intensity with a spacing of about 13 G (1.3 mT).[4][5][6]
The inorganic aminoxyl group is a persistent radical, akin to TEMPO.
It has been used in some oxidation reactions, such as for oxidation of some anilines and phenols[7][8][9][10][11] allowing polymerization and cross-linking of peptides and peptide-based hydrogels.[12][13]
It can also be used as a model for peroxyl radicals in studies that examine the antioxidant mechanism of action in a wide range of natural products.[14]
Preparation
Frémy's salt is prepared from hydroxylaminedisulfonic acid. Oxidation of the conjugate base gives the purple dianion:
- HON(SO3H)2 → [HON(SO3)2]2− + 2 H+
- 2 [HON(SO3)2]2− + PbO2 → 2 [ON(SO3)2]2− + PbO + H2O
The synthesis can be performed by combining nitrite and bisulfite to give the hydroxylaminedisulfonate. Oxidation is typically conducted at low-temperature, either chemically or by electrolysis.[3][2]
Other reactions:
- HNO2 + 2 HSO−3 → HON(SO3)2−2 + H2O
- 3 HON(SO3)2−2 + MnO−4 + H+ → 3 ON(SO3)2−2 + MnO2 + 2 H2O
- 2 ON(SO3)2−2 + 4 K+ → K4[ON(SO3)2]2
History
Frémy's salt was discovered in 1845 by Edmond Frémy (1814–1894).[15] Its use in organic synthesis was popularized by Hans Teuber, such that an oxidation using this salt is called the Teuber reaction.[9][10]
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ 2.0 2.1 "Synthesis and Characterization of Potassium Nitrosodisulfonate, Frémy's Salt". tripod.com. http://chemistris.tripod.com/science/synthesis_of_fremys_salt.pdf.
- ↑ 3.0 3.1 "Oxidation with the nitrosodisulfonate radical. I. Preparation and use of sodium nitrosodisulfonate: trimethyl-p-benzoquinone". Organic Syntheses 52: 83. 1972. doi:10.15227/orgsyn.052.0083.
- ↑ Electron Spin Resonance: Elementary Theory and Practical Applications. New York: McGraw-Hill. 1972. ISBN 978-0-07-069454-5. See page 463 for information on intensity measurements and page 86 for an EPR spectrum of Frémy's salt.
- ↑ "EPR study of Frémy's salt nitroxide reduction by ascorbic acid; influence of bulk pH values". Res. Chem. Intermed. 26 (9): 885–896. 2000. doi:10.1163/156856700X00372.
- ↑ "Mechanistic similarities between oxidation of hydroethidine by Frémy's salt and superoxide: stopped-flow optical and EPR studies". Free Radical Biology & Medicine 39 (7): 853–863. October 2005. doi:10.1016/j.freeradbiomed.2005.05.001. PMID 16140206.
- ↑ "Oxidations with potassium nitrosodisulfonate (Frémy's radical). Teuber reaction.". Chemical Reviews 71 (2): 229–246. 1971. doi:10.1021/cr60270a005.
- ↑ "Structure-activity studies of antitumor agents based on pyrrolo[1,2-a]benzimidazoles: new reductive alkylating DNA cleaving agents". Journal of Medicinal Chemistry 34 (10): 2954–2961. October 1991. doi:10.1021/jm00114a003. PMID 1920349.
- ↑ 9.0 9.1 "Reaktionen mit Nitrosodisulfonat, XXXVI. Chinolin-chinone-(5.6) aus 5-Hydroxy-chinolinen" (in German). Chem. Ber. 100 (9): 2918–2929. 1967. doi:10.1002/cber.19671000916. http://www3.interscience.wiley.com/cgi-bin/fulltext/112293483/PDFSTART.[yes|permanent dead link|dead link}}]
- ↑ 10.0 10.1 "Use of Dipotassium Nitrosodisulfonate (Frémy's Salt): 4,5-Dimethyl-o-Benzoquinone". Org. Synth. 52: 88. 1972. doi:10.15227/orgsyn.052.0088.
- ↑ "A metabolic activation mechanism of 7H-dibenzo[c,g]carbozole via o-quinone. Part 1: synthesis of 7H-dibenzo[c,g]carbozole-3,4-dione and reactions with nucleophiles". Polycyclic Aromatic Compounds 22 (3–4): 295–300. 2002. doi:10.1080/10406630290026957.
- ↑ "Mussel-inspired new approach for polymerization and cross-linking of peptides and proteins containing tyrosines by Frémy's salt oxidation". Bioconjugate Chemistry 26 (3): 502–510. March 2015. doi:10.1021/bc5006152. PMID 25692389.
- ↑ "Utilizing Frémy's Salt to Increase the Mechanical Rigidity of Supramolecular Peptide-Based Gel Networks" (in English). Frontiers in Bioengineering and Biotechnology 8: 594258. 2021. doi:10.3389/fbioe.2020.594258. PMID 33469530.
- ↑ "Stopped-flow ESR study on the reactivity of vitamin E, vitamin C and its lipophilic derivatives towards Frémy's salt in micellar systems". Chemistry and Physics of Lipids 56 (1): 73–80. November 1990. doi:10.1016/0009-3084(90)90090-E. PMID 1965427.
- ↑ See:
- Frémy, E. (1845) "Sur un nouvelle série d'acides formés d'oxygène, de soufre, d'hydrogène et de d'azote" (On a new series of acids formed from oxygen, sulfur, hydrogen, and nitrogen), Annales de Chimie et de Physique, 3rd series, 15 : 408-488. Frémy's salt appears on p. 447, where it's called "sulfazidate de potasse".
- Frémy, E. (1845) "Sur un nouvelle série d'acides formés d'oxygène, de soufre, d'hydrogène et de d'azote" (On a new series of acids formed from oxygen, sulfur, hydrogen, and nitrogen), Comptes rendus, 21 : 218–226. This is a condensed version of the article that appeared in Annales de Chimie et de Physique.
- "Séances académiques," L'Institut, no. 604, 23 July 1845, pp. 265–266.
- "Séances académiques," L'Institut, no. 619, 12 November 1845, pp. 393. Here a committee of the French Academy of Sciences reviewed Frémy's findings.
- Edward Divers and Tamemasa Haga (1900) "Identification and constitution of Frémy's sulphazotised salts of potassium," Journal of the Chemical Society, Transactions, 77 : 440-446. doi:10.1039/CT9007700440 Here, correct formulae for Frémy's salts are presented. On p. 445 , the salt that Frémy called sulfazidate is identified as ON(SO3K)2.
Further reading
- "Undergraduate Experiments with a Long-Lived Radical (Frémy's salt): Synthesis of 1,4-Benzoquinones by Degradative Oxidation of p-Hydroxybenzyl Alcohols". Journal of Chemical Education 65 (7): 627–629. 1988. doi:10.1021/ed065p627. Bibcode: 1988JChEd..65..627M.
 |

