Chemistry:Furan-2-ylmethanethiol
From HandWiki
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
(Furan-2-yl)methanethiol | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 383594 | |
| ChemSpider | |
| EC Number |
|
| MeSH | furfuryl+mercaptan |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3336 |
| |
| |
| Properties | |
| C5H6OS | |
| Molar mass | 114.16 g·mol−1 |
| Appearance | Colourless liquid |
| Odor | Roasted coffee, Caramel, Sulfurous, Waxy |
| Density | 1.132 g cm−3 |
| Boiling point | 155 °C; 311 °F; 428 K |
| Vapor pressure | 531 Pa |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | WARNING |
| H226 | |
| Flash point | 45 °C (113 °F; 318 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
100-200 mg kg−1 (mouse) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
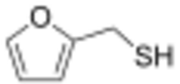
Furan-2-ylmethanethiol (2-Furanmethanethiol) is an organic compound containing a furan substituted with a sulfanylmethyl group. It is a clear colourless liquid when pure, but it becomes yellow coloured upon prolonged standing. It possesses a strong odour of roasted coffee and a bitter taste. It is a key component of the aroma of roasted coffee. It has been identified as a trigger molecule for parosmia following COVID-19 infection.[1][2]
Synthesis
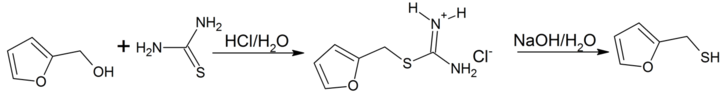
Furan-2-ylmethanethiol is easily prepared by reacting furfuryl alcohol with thiourea in hydrochloric acid via an intermediate isothiouronium salt which is hydrolized to the thiol by heating with sodium hydroxide.[3]
References
- ↑ Parker JK, Kelly CE, Gane SB (5 February 2021). "Molecular Mechanism of Parosmia". p. 21251085. medRxiv 10.1101/2021.02.05.21251085.
- ↑ "Scientists identify 'trigger molecule' for Covid-related changes to smell.". The Guardian. 25 May 2022. https://www.theguardian.com/world/2022/may/25/scientists-identify-trigger-molecule-for-covid-related-changes-to-smell.
- ↑ "Preparation of furfuryl mercaptane". Organic Syntheses 35: 66. 1955. doi:10.15227/orgsyn.035.0066. http://www.orgsyn.org/demo.aspx?prep=CV4P0491.
 |
