Chemistry:Gramine
From HandWiki
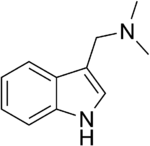
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-(1H-Indol-3-yl)-N,N-dimethylmethanamine | |
| Other names
donaxine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C11H14N2 | |
| Molar mass | 174.24 g/mol |
| Melting point | 138 to 139 °C (280 to 282 °F; 411 to 412 K) |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Gramine (also called donaxine) is a naturally occurring indole alkaloid present in several plant species. Gramine may play a defensive role in these plants, since it is toxic to many organisms.[1]
Occurrence
Gramine has been found in the giant reed, Arundo donax,[2][3] Acer saccharinum (Silver Maple),[4] Hordeum,[1][3] (a grass genus that includes barley) and Phalaris[3] (another grass genus).
Effects and toxicity
Gramine has been found to act as an agonist of the adiponectin receptor 1 (AdipoR1).[5]
The LD50 of gramine is 44.6 mg/ kg iv in mice and 62.9 mg/ kg iv in rats.[6] Numerous studies have been done on the toxicity of gramine to insects harmful to crops in order to assess its potential use as an insecticide.[7]
References
- ↑ 1.0 1.1 Corcuera, L. J. (1993). "Biochemical Basis of the Resistance of the Barley to Aphids". Phytochemistry 33 (4): 741–747. doi:10.1016/0031-9422(93)85267-U.
- ↑ Orechoff, A.; Norkina, S. (1935). "Über die Alkaloide von Arundo Donax L.". Berichte der Deutschen Chemischen Gesellschaft 68 (3): 436–437. doi:10.1002/cber.19350680312.
- ↑ 3.0 3.1 3.2 Cheeke, P. R. (1989). Toxicants of Plant Origin, Alkaloids. CRC Press. p. 172. ISBN 0-8493-6990-8. https://books.google.com/books?id=eASgQyXq8xMC&pg=PA172.
- ↑ Pachter, I. J.; Zacharias, D. E.; Ribeiro, O. (1959). "Indole Alkaloids of Acer saccharinum (the Silver Maple), Dictyoloma incanescens, Piptadenia colubrina, and Mimosa hostilis". Journal of Organic Chemistry 24 (9): 1285–1287. doi:10.1021/jo01091a032.
- ↑ "Identification of adiponectin receptor agonist utilizing a fluorescence polarization based high throughput assay". PLOS ONE 8 (5): e63354. 2013. doi:10.1371/journal.pone.0063354. PMID 23691032. Bibcode: 2013PLoSO...863354S.
- ↑ Erspamer, V. (1954). "Pharmacology of Indolealkylamines". Pharmacological Reviews 6 (4): 425–487. PMID 13236482. http://pharmrev.aspetjournals.org/content/6/4/425.abstract.
- ↑ Corcuera, L. J. (1984). "Effects of Indole Alkaloids from Gramineae on Aphids". Phytochemistry 23 (3): 539–541. doi:10.1016/S0031-9422(00)80376-3.
 |

