Chemistry:Heptasulfur imide
| |
| |
| Names | |
|---|---|
| IUPAC name
Azacyclooctasulfane
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| S 7NH | |
| Molar mass | 239.44 g·mol−1 |
| Appearance | Pale yellow solid |
| Density | 2.01 g/cm3 |
| Melting point | 113.5 °C (236.3 °F; 386.6 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
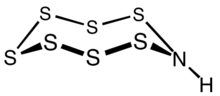
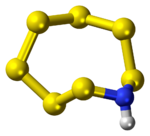
Heptasulfur imide is the inorganic compound with the formula S
7NH. It is a pale yellow solid that is, like elemental sulfur, highly soluble in carbon disulfide. The compound, which is only of academic interest, is representative of a family of sulfur imides (or azacyclosulfanes or thiacycloazanes) S
x(NH)
y.
Synthesis and structure
It is prepared by reaction of disulfur dichloride with ammonia,[1] although other methods have been developed.[2] Together with S
7NH, the reaction also produces three isomers of S
6(NH)
2 (diazacyclooctasulfanes) and two isomers of S
5(NH)
3 (triazacyclooctasulfanes).[which?]
Azacyclooctasulfane is an analogue of octasulfur (cyclooctasulfane) S
8, with one –S– replaced by –NH–. The S–NH–S center is almost planar,[3] suggesting that the amine is nonbasic.
References
- ↑ Becke-Goehring, Margot; Fluck, Ekkehard "Heptasulfur imide" Inorganic Syntheses 1966, vol. 8, 103-5. doi:10.1002/9780470132395.ch26
- ↑ Bojes, J.; Chivers, T.; Drummond, I. "Heptathiazocine(heptasulfurimide) and tetrabutylammonium tetrathionitrate" Inorganic Syntheses (1978), 18, 203-6. doi:10.1002/9780470132494.ch36
- ↑ Hecht, H. J.; Reinhardt, R.; Steudel, R.; Bradaczek, H. "Redetermination of the crystal and molecular structure of heptasulfur imide, S7NH" Zeitschrift für Anorganische und Allgemeine Chemie 1976, vol. 426, pp. 43-8. doi:10.1002/zaac.19764260106
 |

