Chemistry:Disulfur dichloride
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Disulfur dichloride
| |||
| Systematic IUPAC name
Dichlorodisulfane | |||
Other names
| |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| DrugBank | |||
| EC Number |
| ||
| MeSH | Sulfur+monochloride | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 3390 | ||
| |||
| |||
| Properties | |||
| S 2Cl 2 | |||
| Molar mass | 135.02 g·mol−1 | ||
| Appearance | Light-amber to yellow-red, oily liquid[1] | ||
| Odor | pungent, nauseating, irritating[1] | ||
| Density | 1.688 g/cm3 | ||
| Melting point | −80 °C (−112 °F; 193 K) | ||
| Boiling point | 137.1 °C (278.8 °F; 410.2 K) | ||
| Decomposes, with loss of HCl | |||
| Solubility | Soluble in ethanol, benzene, ether, THF, chloroform, CCl 4[2] | ||
| Vapor pressure | 7 mmHg (20 °C)[1] | ||
| −62.2·10−6 cm3/mol | |||
Refractive index (nD)
|
1.658 | ||
| Structure | |||
| C2 | |||
| 2 at sulfur atoms | |||
| gauche | |||
| 1.60 D[2] | |||
| Hazards | |||
| Safety data sheet | ICSC 0958 | ||
| GHS pictograms |    
| ||
| GHS Signal word | Danger | ||
| H301, H314, H332, H400 | |||
| P260, P261, P264, P270, P271, P273, P280, P301+310, P301+330+331, P303+361+353, P304+312, P304+340, P305+351+338, P310, P312, P321, P330, P363, P391, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 118.5 °C (245.3 °F; 391.6 K) | ||
| 234 °C (453 °F; 507 K) | |||
| Lethal dose or concentration (LD, LC): | |||
LCLo (lowest published)
|
150 ppm (mouse, 1 min) (1 ppm = 5.52 mg/m3)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 1 ppm (5.52 mg/m3)[1] | ||
REL (Recommended)
|
C 1 ppm (5.52 mg/m3)[1] | ||
IDLH (Immediate danger)
|
5 ppm[1] (1 ppm = 5.52 mg/m3) | ||
| Related compounds | |||
Related sulfur chlorides/oxychlorides
|
|||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Disulfur dichloride (or disulphur dichloride by the British English spelling) is the inorganic compound of sulfur and chlorine with the formula S
2Cl
2.[4][5][6][7] It is an amber oily liquid.
Sometimes, this compound is incorrectly named sulfur monochloride (or sulphur monochloride by the British English spelling), the name implied by its empirical formula SCl.
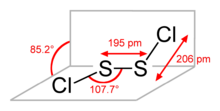
S
2Cl
2 has the structure implied by the formula Cl–S–S–Cl, wherein the dihedral angle between the Cla
–S–S and S–S–Clb
planes is 85.2°. This structure is referred to as gauche, and is akin to that for H
2O
2. A rare isomer of S
2Cl
2 is S=SCl
2 (thiothionyl chloride); this isomer forms transiently when S
2Cl
2 is exposed to UV-radiation (see thiosulfoxides).
Synthesis, basic properties, reactions
Disulfur dichloride is a yellow liquid that fumes in moist air due to reaction with water:
- 16 S
2Cl
2 + 16 H
2O → 8 SO
2 + 32 HCl + 3 S
8
It is produced by partial chlorination of elemental sulfur. The reaction proceeds at usable rates at room temperature. In the laboratory, chlorine gas is led into a flask containing elemental sulfur. As disulfur dichloride is formed, the contents become a golden yellow liquid:[8]
- S
8 + 4 Cl
2 → 4 S
2Cl
2, ΔH = −58.2 kJ/mol
Excess chlorine produces sulfur dichloride, which causes the liquid to become less yellow and more orange-red:
- S
2Cl
2 + Cl
2 ⇌ 2 SCl
2, ΔH = −40.6 kJ/mol
The reaction is reversible, and upon standing, SCl
2 releases chlorine to revert to the disulfur dichloride. Disulfur dichloride has the ability to dissolve large quantities of sulfur, which reflects in part the formation of polysulfanes:
- 8 S
2Cl
2 + n S
8 → 8 S
n+2Cl
2
Disulfur dichloride can be purified by distillation from excess elemental sulfur.
S
2Cl
2 also arises from the chlorination of CS
2 as in the synthesis of thiophosgene or carbon tetrachloride.
Reactions
S
2Cl
2 hydrolyzes to sulfur dioxide and elemental sulfur. When treated with hydrogen sulfide, polysulfanes are formed as indicated in the following idealized formula:
- 2 H
2S + S
2Cl
2 → H
2S
4 + 2 HCl
It reacts with ammonia to give heptasulfur imide (S
7NH) and related S−N rings S
8-n(NH)
n (n = 2, 3).
Applications
S
2Cl
2 has been used to introduce C−S bonds. In the presence of aluminium chloride (AlCl
3), S
2Cl
2 reacts with benzene to give diphenyl sulfide:
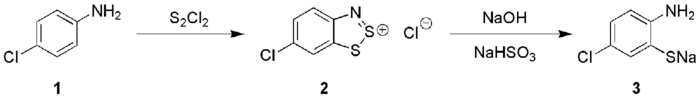
Anilines (1) react with S
2Cl
2 in the presence of NaOH to give 1,2,3-benzodithiazolium chloride (2) (Herz reaction) which can be transformed into ortho-aminothiophenolates (3), these species are precursors to thioindigo dyes.
It is also used to prepare mustard gas via ethylene at 60 °C (the Levinstein process):
- 8 S
2Cl
2 + 16 H
2C=CH
2 → 8 (ClCH
2CH
2)
2S + S
8
Other uses of S
2Cl
2 include the manufacture of sulfur dyes, insecticides, and synthetic rubbers. It is also used in cold vulcanization of rubbers, as a polymerization catalyst for vegetable oils and for hardening soft woods.[9]
Safety and regulation
S
2Cl
2 can be used to produce bis(2-chloroethyl)sulfide S(CH
2CH
2Cl)
2, known as the mustard gas:[9]
Consequently, it is listed in Schedule 3 of the Chemical Weapons Convention. Facilities that produce and/or process and/or consume scheduled chemicals may be subject to control, reporting mechanisms and inspection by the Organisation for the Prohibition of Chemical Weapons.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 NIOSH Pocket Guide to Chemical Hazards. "#0578". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0578.html.
- ↑ 2.0 2.1 Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN:0-07-049439-8
- ↑ "Sulfur monochloride". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/10025679.html.
- ↑ Holleman, A. F.; Wiberg, E. Inorganic Chemistry Academic Press: San Diego, 2001. ISBN:0-12-352651-5.
- ↑ Hartman, W. W.; Smith, L. A.; Dickey, J. B. (1934). "Diphenylsulfide". Organic Syntheses 14: 36. http://www.orgsyn.org/demo.aspx?prep=cv2p0242.; Collective Volume, 2, pp. 242
- ↑ R. J. Cremlyn An Introduction to Organosulfur Chemistry John Wiley and Sons: Chichester (1996). ISBN:0-471-95512-4
- ↑ Garcia-Valverde M., Torroba T. (2006). "Heterocyclic chemistry of sulfur chlorides – Fast ways to complex heterocycles". European Journal of Organic Chemistry 2006 (4): 849–861. doi:10.1002/ejoc.200500786.
- ↑ F. Fehér "Dichlorodisulfane" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 371.
- ↑ 9.0 9.1 Lauss, Hans-Dietrich; Steffens, Wilfried (2000). "Sulfur Halides". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a25_623. ISBN 3527306730.
 |