Chemistry:Hexafluorobutadiene
From HandWiki
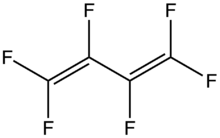
| |
| Names | |
|---|---|
| Preferred IUPAC name
Hexafluorobuta-1,3-diene | |
| Other names
1,1,2,3,4,4-Hexafluoro-1,3-butadiene, FC 2316
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C4F6 | |
| Molar mass | 162.034 g·mol−1 |
| Appearance | colorless gas |
| Density | 1.44 g/cm3 (@15 °C) |
| Melting point | −132 °C (−206 °F; 141 K) |
| Boiling point | 6 °C (43 °F; 279 K) |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H220, H280, H331 | |
| P210, P261, P271, P304+340, P311, P321, P377, P381, P403, P403+233, P405, P410+403, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hexafluorobutadiene is an organofluorine compound with the formula (CF2=CF)2. A colorless gas, it has attracted attention as an etchant in microelectronics. It is the perfluoroanalogue of butadiene.
It can be prepared by coupling of C2 compounds such as from chlorotrifluoroethylene or bromotrifluoroethylene. Routes from C4 species have also been demonstrated. For example, an early synthesis involved Zn-induced dechlorinaion of 1,2,3,4-tetrachloro-1,1,2,3,4,4-hexafluorobutane.[citation needed]
Hexafluorobutadiene dimerizes via a [2+2] process at 150 °C to give perfluorinated divinylcyclobutanes.[1]
See also
- Hexafluoro-2-butyne, an isomer of C4F6
- Hexafluorocyclobutene, an isomer of C4F6
- Hexachlorobutadiene
References
- ↑ Lemal, David M.; Chen, Xudong (2005). "Fluorinated Cyclobutanes and Their Derivatives". in Zvi Rappoport. The Chemistry of Cyclobutanes. PATAI'S Chemistry of Functional Groups. pp. 955–1029. doi:10.1002/0470864028.ch21. ISBN 0470864001.
 |

