Chemistry:Hexamminecobalt(III) chloride
| |

| |
| Names | |
|---|---|
| IUPAC name
Hexaamminecobalt(III) chloride
| |
| Other names
Cobalt hexammine chloride, hexaamminecobalt(III) chloride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| H18N6Cl3Co | |
| Molar mass | 267.48 g/mol |
| Appearance | yellow or orange crystals |
| Density | 1.71 g/cm3, |
| Melting point | decomposes |
| 0.26 M (20 °C) tribromide: 0.04 M (18 °C) | |
| Solubility | soluble in NH3 |
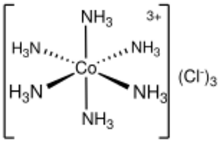
| Structure | |
| octahedral | |
| 0 D | |
| Hazards | |
| Main hazards | poison |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
| Related compounds | |
Other anions
|
[Co(NH3)6]Br3 [Co(NH3)6](OAc)3 |
Other cations
|
[Cr(NH3)6]Cl3 [Ni(NH3)6]Cl2 |
Related compounds
|
[Co(H2NCH2CH2NH2)3]Cl3 [Co(NH3)5(H2O)]Cl3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hexaamminecobalt(III) chloride is the chemical compound with the formula [Co(NH3)6]Cl3. It is the chloride salt of the coordination complex [Co(NH3)6]3+, which is considered an archetypal "Werner complex", named after the pioneer of coordination chemistry, Alfred Werner. The cation itself is a metal ammine complex with six ammonia ligands attached to the cobalt(III) ion.
Originally salts of [Co(NH3)6]3+ were described as the luteo (Latin: yellow) complex of cobalt. This name has been discarded as modern chemistry considers color less important than molecular structure. Other similar complexes also had color names, such as purpureo (Latin: purple) for a cobalt pentammine complex, and praseo (Greek: green) and violeo (Latin: violet) for two isomeric tetrammine complexes.[1]
Properties and structure
[Co(NH3)6]3+ is diamagnetic, with a low-spin 3d6 octahedral Co(III) center. The cation obeys the 18-electron rule and is considered to be a classic example of an exchange inert metal complex. As a manifestation of its inertness, [Co(NH3)6]Cl3 can be recrystallized unchanged from concentrated hydrochloric acid: the NH3 is so tightly bound to the Co(III) centers that it does not dissociate to allow its protonation.[2] In contrast, labile metal ammine complexes, such as [Ni(NH3)6]Cl2, react rapidly with acids, reflecting the lability of the Ni(II)–NH3 bonds. Upon heating, hexamminecobalt(III) begins to lose some of its ammine ligands, eventually producing a stronger oxidant.
The chloride ions in [Co(NH3)6]Cl3 can be exchanged with a variety of other anions such as nitrate, bromide, iodide, sulfamate to afford the corresponding [Co(NH3)6]X3 derivative. Such salts are orange or bright yellow and display varying degrees of water solubility. The chloride ion can be also exchanged with more complex anions such as the hexathiocyanatochromate(III), yielding a pink compound with formula [Co(NH3)6][Cr(SCN)6], or the ferricyanide ion.
Preparation
[Co(NH3)6]Cl3 is prepared by treating cobalt(II) chloride with ammonia and ammonium chloride followed by oxidation. Oxidants include hydrogen peroxide or oxygen in the presence of charcoal catalyst.[2] This salt appears to have been first reported by Fremy.[3]
The acetate salt can be prepared by aerobic oxidation of cobalt(II) acetate, ammonium acetate, and ammonia in methanol.[4] The acetate salt is highly water-soluble to the level of 1.9 M (20 °C), versus 0.26 M for the trichloride.
Uses
[Co(NH3)6]3+ is a component of some structural biology methods (especially for DNA or RNA, where positive ions stabilize tertiary structure of the phosphate backbone), to help solve their structures by X-ray crystallography[5] or by nuclear magnetic resonance.[6] In the biological system, the counterions would more probably be Mg2+, but the heavy atoms of cobalt (or sometimes iridium, as in PDB: 2GIS) provide anomalous scattering to solve the phase problem and produce an electron-density map of the structure.[7]
[Co(NH3)6]3+ is an unusual example of a water-soluble trivalent metal complex and is of utility for charge-shielding applications such as the stabilization of highly negatively charged complexes, such as interactions with and between nucleic acids. The compound induced the transition of DNA structure from the classical B-form to the Z-form.[8]
Related compounds
References
- ↑ Huheey, James E. (1983). Inorganic Chemistry (3rd ed.). p. 360.
- ↑ Jump up to: 2.0 2.1 Bjerrum, J.; McReynolds, J. P. (1946). "Hexamminecobalt(III) Salts". Inorg. Synth. 2: 216–221. doi:10.1002/9780470132333.ch69.
- ↑ Fremy, M. E. (1852). "Recherches sur le cobalt". Ann. Chim. Phys. 35: 257–312. http://gallica.bnf.fr/ark:/12148/bpt6k34776q/f255.table.
- ↑ Lindholm, R. D.; Bause, Daniel E. (1978). "Complexes of Cobalt Containing Ammonia or Ethylene Diamine: Hexaamminecobalt(III) Salts". Inorg. Synth. 18: 67–69. doi:10.1002/9780470132494.ch14.
- ↑ Ramakrishnan, B.; Sekharudu, C.; Pan, B.; Sundaralingam, M. (2003). "Near-atomic resolution crystal structure of an A-DNA decamer d(CCCGATCGGG): cobalt hexammine interaction with A-DNA". Acta Crystallogr. D59 (Pt 1): 67–72. doi:10.1107/s0907444902018917. PMID 12499541.
- ↑ Rudisser, S.; Tinoco, I., Jr. (2000). "Solution structure of Cobalt(III)hexammine complexed to the GAAA tetraloop, and metal-ion binding to G.A mismatches.". J. Mol. Biol. 295 (5): 1211–1232. doi:10.1006/jmbi.1999.3421. PMID 10653698.
- ↑ McPherson, Alexander (2002). Introduction to Macromolecular Crystallography. John Wiley & Sons. ISBN 0-471-25122-4.
- ↑ 10.1080/07391102.1986.10508453

