Chemistry:Homocystine
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
(2S,2′S)-4,4'-Disulfanediylbis(2-aminobutanoic acid)
| |
| Other names
L-Homocystine; L-4,4′-Dithiobis(2-aminobutanoic acid)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H16N2O4S2 | |
| Molar mass | 268.35 g·mol−1 |
| Appearance | colorless solid |
| Melting point | 281–284 °C (538–543 °F; 554–557 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
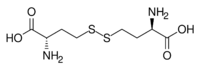
Homocystine is the organosulfur compound with the formula (HO
2CCH(NH
2)CH
2CH
2S)
2. It is disulfide derived from oxidation of homocysteine.[2] Its relationship with homocysteine is analogous to the relationship between cystine and cysteine.
References
- ↑ "L-Homocystine". Sigma-Aldrich. http://www.sigmaaldrich.com/catalog/product/sigma/h6010.
- ↑ Jackson, Peter; Stanley, Keith; Luzio, J. Paul (1986). "Specific fluorescent detection of disulphide-bridged peptides on thin-layer chromatograms". Biochemical Society Transactions 14 (4): 750–751. doi:10.1042/bst0140750.
 |
