Chemistry:Homocysteine
Homocysteine (/ˌhoʊmoʊˈsɪstiːn/; symbol Hcy) is a non-proteinogenic α-amino acid. It is a homologue of the amino acid cysteine, differing by an additional methylene bridge (–CH
2–). It is biosynthesized from methionine by the removal of its terminal Cε methyl group.
Although the production of homocysteine is a normal part of the metabolism of methionine, an excess of homocysteine can be harmful. There are two primary ways for organisms such as humans to metabolize homocysteine: remethylation and transsulfuration.
Remethylation adds a methyl group to the homocysteine molecule, converting homocysteine back into methionine. There are two known remethylation pathways. One pathway requires vitamin B9 (folate) and B12 (cobalamin), which drive the MTR (methionine synthase) and MTRR (methionine synthase reductase) enzymes. The other pathway uses TMG (trimethylglycine) to drive the BHMT (betaine-homocysteine methyltransferase) enzyme.
Transsulfuration converts homocysteine to cystathionine. This pathway requires vitamin B6 to drive the CBS (cystathionine beta synthase) enzyme.[1] Cystathionine is the immediate precursor of the amino acid cysteine, which (along with glutamate and glycine), is incorporated into the tripeptide glutathione, a major antioxidant in the human body.
Homocysteine is therefore an important metabolic substrate. However, excessive levels of homocysteine can result in hyperhomocysteinemia, which is regarded as an indicator of cardiovascular disease risk. Homocysteine likely contributes to atherogenesis, which can result in ischemic injury. Therefore, hyperhomocysteinemia is a possible risk factor for coronary artery disease. Coronary artery disease occurs when an atherosclerotic plaque blocks blood flow to the coronary arteries, which supply the heart with oxygenated blood.[2][3] Hyperhomocysteinemia has also been correlated with the occurrence of blood clots, heart attacks, and strokes, although it is unclear whether hyperhomocysteinemia is an independent risk factor for these conditions.[4] Hyperhomocysteinemia has also been associated with early-term spontaneous abortions[5] and with neural tube defects.[6]

Biosynthesis and biochemical roles
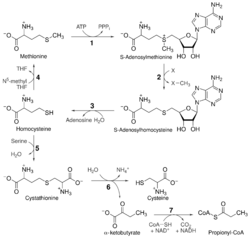
Homocysteine is biosynthesized naturally via a multi-step process.[7] First, methionine receives an adenosine group from ATP, a reaction catalyzed by S-adenosyl-methionine synthetase, to give S-adenosyl methionine (SAM). SAM is widely used source of methyl radicals as a cofactor for radical SAM enzymes. Transfer of the methyl group to an acceptor molecule gives S-adenosyl-homocysteine. Hydrolysis of this thioether gives L-homocysteine. L-Homocysteine reacts with tetrahydrofolate (THF) to give L-methionine.[clarification needed][8]
Biosynthesis of cysteine
Mammals biosynthesize the amino acid cysteine via homocysteine. Cystathionine β-synthase catalyses the condensation of homocysteine and serine to give cystathionine. This reaction uses pyridoxine (vitamin B6) as a cofactor. Cystathionine γ-lyase then converts this double amino acid to cysteine, ammonia, and α-ketobutyrate. Bacteria and plants rely on a different pathway to produce cysteine, relying on O-acetylserine.[9]

Methionine salvage
Homocysteine can be recycled into methionine. This process uses N5-methyl tetrahydrofolate as the methyl donor and cobalamin (vitamin B12)-related enzymes. More detail on these enzymes can be found in the article for methionine synthase.
Other reactions of biochemical significance
Homocysteine can cyclize to give homocysteine thiolactone, a five-membered heterocycle. Because of this "self-looping" reaction, homocysteine-containing peptides tend to cleave themselves by reactions generating oxidative stress.[10]
Homocysteine also acts as an allosteric antagonist at Dopamine D2 receptors.[11]
It has been proposed that both homocysteine and its thiolactone may have played a significant role in the appearance of life on the early Earth.[12]
Homocysteine levels
Homocysteine levels typically are higher in men than women, and increase with age.[13][14]
Common levels in Western populations are 10 to 12 μmol/L, and levels of 20 μmol/L are found in populations with low B-vitamin intakes or in the elderly (e.g., Rotterdam, Framingham).[15][16]
It is decreased with methyl folate trapping, where it is accompanied by decreased methylmalonic acid, increased folate, and a decrease in formiminoglutamic acid.[17] This is the opposite of MTHFR C677T mutations, which result in an increase in homocysteine.
| Sex | Age | Lower limit | Upper limit | Unit | Elevated | Therapeutic target |
| Female | 12–19 years | 3.3[18] | 7.2[18] | μmol/L | > 10.4 μmol/L or > 140 μg/dl |
< 6.3 μmol/L[19] or < 85 μg/dL[19] |
| 45[20] | 100[20] | μg/dL | ||||
| >60 years | 4.9[18] | 11.6[18] | μmol/L | |||
| 66[20] | 160[20] | μg/dL | ||||
| Male | 12–19 years | 4.3[18] | 9.9[18] | μmol/L | > 11.4 μmol/L or > 150 μg/dL | |
| 60[20] | 130[20] | μg/dL | ||||
| >60 years | 5.9[18] | 15.3[18] | μmol/L | |||
| 80[20] | 210[20] | μg/dL |
The ranges above are provided as examples only; test results always should be interpreted using the range provided by the laboratory that produced the result.
Elevated homocysteine
Abnormally high levels of homocysteine in the serum, above 15 μmol/L, are a medical condition called hyperhomocysteinemia.[21] This has been claimed to be a significant risk factor for the development of a wide range of diseases, in total more than 100[22] including thrombosis,[23] neuropsychiatric illness,[24][25][26][27] in particular dementia[28] and fractures.[29][30] It also is found to be associated with microalbuminuria (moderately increased albuminuria), which is a strong indicator of the risk of future cardiovascular disease and renal dysfunction.[31] Vitamin B12 deficiency, even when coupled with high serum folate levels, has been found to increase overall homocysteine concentrations as well.[32]
Typically, hyperhomocysteinemia is managed with vitamin B6, vitamin B9, and vitamin B12 supplementation.[33] However, supplementation with these vitamins does not appear to improve cardiovascular disease outcomes.[34]
References
- ↑ "Homocysteine". https://dmec.moh.gov.vn/documents/10182/31934092/upload_00034511_1656320270953.pdf?version=1.0&fileId=31960513.
- ↑ Kim J, Kim H, Roh H, Kwon Y (2018). "Causes of hyperhomocysteinemia and its pathological significance.". Arch Pharm Res 41 (4): 372–383. doi:10.1007/s12272-018-1016-4. PMID 29552692.
- ↑ Boudi, Brian F. "Noncoronary Atherosclerosis". Medscape. http://emedicine.medscape.com/article/1950759-overview#aw2aab6b3.
- ↑ Homocysteine: The Facts, Tufts Health and Nutrition Letter, July 31, 2020 update
- ↑ "Homocysteine and folate levels as risk factors for recurrent early pregnancy loss". Obstet Gynecol 95 (4): 519–24. 2000. doi:10.1016/s0029-7844(99)00610-9. PMID 10725483.
- ↑ van der Put NJ et al Folate, Homocysteine and Neural Tube Defects: An Overview Exp Biol Med (Maywood) April 2001 vol. 226 no. 4 243-270
- ↑ Selhub, J. (1999). "Homocysteine metabolism". Annual Review of Nutrition 19: 217–246. doi:10.1146/annurev.nutr.19.1.217. PMID 10448523.
- ↑ Champe, PC and Harvey, RA. "Biochemistry. Lippincott's Illustrated Reviews" 4th ed. Lippincott Williams and Wilkins, 2008
- ↑ Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.
- ↑ "Homocystamides promote free-radical and oxidative damage to proteins". Proc. Natl. Acad. Sci. U.S.A. 107 (2): 551–4. January 2010. doi:10.1073/pnas.0909737107. PMID 20080717. Bibcode: 2010PNAS..107..551S.
- ↑ Agnati, LF; Ferré, S; Genedani, S; Leo, G; Guidolin, D; Filaferro, M; Carriba, P; Casadó, V et al. (Nov 2006). "Allosteric modulation of dopamine D2 receptors by homocysteine". Journal of Proteome Research 5 (11): 3077–83. doi:10.1021/pr0601382. PMID 17081059. http://www.cigs.unimo.it/cigsdownloads/labs/cnf_sp2/pubblicazioni/19-agnati%20allosteric%20modulation.pdf.
- ↑ Vallee, Yannick; Shalayel, Ibrahim; Ly, Kieu-Dung; Rao, K. V. Raghavendra; Paëpe, Gael De; Märker, Katharina; Milet, Anne (2017-11-08). "At the very beginning of life on Earth: the thiol-rich peptide (TRP) world hypothesis". International Journal of Developmental Biology 61 (8–9): 471–478. doi:10.1387/ijdb.170028yv. ISSN 0214-6282. PMID 29139533. http://www.ijdb.ehu.es/web/paper/170028yv/at-the-very-beginning-of-life-on-earth-the-thiol-rich-peptide-trp-world-hypothesis.
- ↑ Nygård, O; Vollset, SE; Refsum, H; Stensvold, I; Tverdal, A; Nordrehaug, JE; Ueland, M; Kvåle, G (Nov 15, 1995). "Total plasma homocysteine and cardiovascular risk profile. The Hordaland Homocysteine Study". JAMA: The Journal of the American Medical Association 274 (19): 1526–33. doi:10.1001/jama.274.19.1526. PMID 7474221.
- ↑ Refsum, H; Nurk, E; Smith, AD; Ueland, PM; Gjesdal, CG; Bjelland, I; Tverdal, A; Tell, GS et al. (June 2006). "The Hordaland Homocysteine Study: a community-based study of homocysteine, its determinants, and associations with disease". The Journal of Nutrition 136 (6 Suppl): 1731S–1740S. doi:10.1093/jn/136.6.1731S. PMID 16702348.
- ↑ Bots, Michiel L.; Launer, Lenore J.; Lindemans, Jan; Hoes, Arno W.; Hofman, Albert; Witteman, Jacqueline C. M.; Koudstaal, Peter J.; Grobbee, Diederick E. (1999-01-11). "Homocysteine and Short-term Risk of Myocardial Infarction and Stroke in the Elderly". Archives of Internal Medicine 159 (1): 38–44. doi:10.1001/archinte.159.1.38. ISSN 0003-9926. PMID 9892328.
- ↑ Selhub, J.; Jacques, P. F.; Bostom, A. G.; Wilson, P. W.; Rosenberg, I. H. (2000). "Relationship between plasma homocysteine and vitamin status in the Framingham study population. Impact of folic acid fortification". Public Health Reviews 28 (1–4): 117–145. ISSN 0301-0422. PMID 11411265.
- ↑ Scott, JohnM.; Weir, DonaldG. (15 August 1981). "THE METHYL FOLATE TRAP: A physiological response in man to prevent methyl group deficiency in kwashiorkor (methionine deficiency) and an explanation for folic-acid-induced exacerbation of subacute combined degeneration in pernicious anaemia". The Lancet 318 (8242): 337–340. doi:10.1016/S0140-6736(81)90650-4. ISSN 0140-6736. PMID 6115113.
- ↑ 18.0 18.1 18.2 18.3 18.4 18.5 18.6 18.7 "Homocysteine". http://www.thedoctorsdoctor.com/labtests/homocysteine.htm.
- ↑ 19.0 19.1 Adëeva Nutritionals Canada > Optimal blood test values Retrieved on July 9, 2009
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 20.6 20.7 Derived from molar values using molar massof 135 g/mol
- ↑ "Hyperhomocysteinemia - Hematology and Oncology - Merck Manuals Professional Edition". http://www.merckmanuals.com/professional/hematology-and-oncology/thrombotic-disorders/hyperhomocysteinemia#v12779073.
- ↑ Smith, A. D.; Refsum, H. (October 2021). "Homocysteine – from disease biomarker to disease prevention". Journal of Internal Medicine 290 (4): 826–854. doi:10.1111/joim.13279. ISSN 0954-6820. PMID 33660358. https://onlinelibrary.wiley.com/doi/10.1111/joim.13279.
- ↑ Cattaneo, M (February 1999). "Hyperhomocysteinemia, atherosclerosis and thrombosis". Thrombosis and Haemostasis 81 (2): 165–76. doi:10.1055/s-0037-1614438. PMID 10063987.
- ↑ Morris, MS (July 2003). "Homocysteine and Alzheimer's disease". Lancet Neurology 2 (7): 425–8. doi:10.1016/s1474-4422(03)00438-1. PMID 12849121.
- ↑ Smach, MA; Jacob, N; Golmard, JL; Charfeddine, B; Lammouchi, T; Ben Othman, L; Dridi, H; Bennamou, S et al. (2011). "Folate and homocysteine in the cerebrospinal fluid of patients with Alzheimer's disease or dementia: a case control study". European Neurology 65 (5): 270–8. doi:10.1159/000326301. PMID 21474939.
- ↑ Smith, AD; Smith, SM; de Jager, CA; Whitbread, P; Johnston, C; Agacinski, G; Oulhaj, A; Bradley, KM et al. (Sep 8, 2010). "Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial". PLOS ONE 5 (9). doi:10.1371/journal.pone.0012244. PMID 20838622. Bibcode: 2010PLoSO...512244S.
- ↑ Dietrich-Muszalska, A; Malinowska, J; Olas, B; Głowacki, R; Bald, E; Wachowicz, B; Rabe-Jabłońska, J (May 2012). "The oxidative stress may be induced by the elevated homocysteine in schizophrenic patients". Neurochemical Research 37 (5): 1057–62. doi:10.1007/s11064-012-0707-3. PMID 22270909.
- ↑ Smith, A. David; Refsum, Helga; Bottiglieri, Teodoro; Fenech, Michael; Hooshmand, Babak; McCaddon, Andrew; Miller, Joshua W.; Rosenberg, Irwin H. et al. (2018). "Homocysteine and Dementia: An International Consensus Statement". Journal of Alzheimer's Disease 62 (2): 561–570. doi:10.3233/JAD-171042. ISSN 1387-2877. PMID 29480200. PMC 5836397. https://www.researchwithrutgers.com/en/publications/homocysteine-and-dementia-an-international-consensus-statement.
- ↑ McLean, RR; Jacques, PF; Selhub, J; Tucker, KL; Samelson, EJ; Broe, KE; Hannan, MT; Cupples, LA et al. (May 13, 2004). "Homocysteine as a predictive factor for hip fracture in older persons". The New England Journal of Medicine 350 (20): 2042–9. doi:10.1056/NEJMoa032739. PMID 15141042.
- ↑ van Meurs, JB; Dhonukshe-Rutten, RA; Pluijm, SM; van der Klift, M; de Jonge, R; Lindemans, J; de Groot, LC; Hofman, A et al. (May 13, 2004). "Homocysteine levels and the risk of osteoporotic fracture". The New England Journal of Medicine 350 (20): 2033–41. doi:10.1056/NEJMoa032546. PMID 15141041. http://repub.eur.nl/pub/8452.
- ↑ Jager, A; Kostense, PJ; Nijpels, G; Dekker, JM; Heine, RJ; Bouter, LM; Donker, AJ; Stehouwer, CD (Jan 2001). "Serum homocysteine levels are associated with the development of (micro)albuminuria: the Hoorn study". Arteriosclerosis, Thrombosis, and Vascular Biology 21 (1): 74–81. doi:10.1161/01.ATV.21.1.74. PMID 11145936.
- ↑ Selhub, J.; Morris, M. S.; Jacques, P. F. (2007-12-04). "In vitamin B12 deficiency, higher serum folate is associated with increased total homocysteine and methylmalonic acid concentrations". Proceedings of the National Academy of Sciences 104 (50): 19995–20000. doi:10.1073/pnas.0709487104. ISSN 0027-8424. PMID 18056804. Bibcode: 2007PNAS..10419995S.
- ↑ Stehouwer, Coen DA; Guldener, Coen van (2005). "Homocysteine-lowering treatment: An overview". Expert Opinion on Pharmacotherapy 2 (9): 1449–60. doi:10.1517/14656566.2.9.1449. PMID 11585023.
- ↑ Martí-Carvajal, Arturo J.; Solà, Ivan; Lathyris, Dimitrios (15 January 2015). Martí-Carvajal, Arturo J. ed. "Homocysteine-lowering interventions for preventing cardiovascular events". The Cochrane Database of Systematic Reviews 1. doi:10.1002/14651858.CD006612.pub4. ISSN 1469-493X. PMID 25590290.
External links
- Homocysteine MS Spectrum
- Homocysteine at Lab Tests Online
- Prof. David Spence on homocysteine levels, kidney damage, and cardiovascular disease, The Health Report, Radio National, 24 May 2010
 |
