Chemistry:Humanin
 Generic protein structure example |
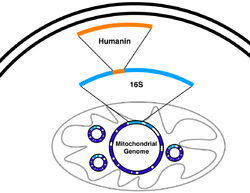
Humanin is a micropeptide encoded in the mitochondrial genome by the 16S ribosomal RNA gene, MT-RNR2. Its structure contains a three-turn α-helix, and no symmetry.
In in vitro and animal models, it appears to have cytoprotective effects.[1][2][3][4]
Gene
Humanin is encoded in the mitochondrial genome by the 16S ribosomal RNA gene, MT-RNR2.[5] Multiple paralogs are found in the nuclear genome (due to nuclear mitochondrial DNA segments) and are named MTRNR2L followed by a number. It is not entirely sure whether these paralogous isoforms are completely unexpressed.[6]
Protein
The expressed peptide[7] contains a three-turn α-helix, and has no symmetry.[7]
The length of the peptide depends on where it is produced. If it is produced inside the mitochondria it will be 21 amino acids long.[8] If it is produced outside the mitochondria, in the cytosol, it will be 24 amino acids long.[8] Both peptides have been shown to have biological activity.[8][9]
Other species
Humanin is the most well-conserved of the mitochondria-derived peptides, found in such diverse species as humans, naked mole rats, and nematodes.[3] Overexpression of humanin in Caenorhabditis elegans has been shown to extend the lifespan of that nematode by increasing autophagy.[3]
The rat, Rattus norvegicus, has a gene, rattin (C0HLU6, "Humanin-like protein"), that encodes a 38 amino acid peptide homologous to humanin.[10] The two genes produce cDNAs that show 88% sequence identity.[10] The peptides are 81% identical, with the carboxyl terminal sequence in rattin being 14 amino acids longer than in humanin.[10] Of the 24 amino acids in the rest of the rat sequence, 20 are identical to the amino acids in the human sequence.[10]
The mouse MT-RNR2 humanin ortholog is a pseudogene, so no humanin is produced from the mtDNA. However, the nuclear genome harbors (like in humans) many copies of mitochondrial genomes, and one copy of the humanin homolog, Gm20594 (J3QJY3), is actively expressed.[11]
Function
Humanin has several cytoprotective effects.[12]
Interactions
Extracellular interaction with a tripartite receptor composed of gp130, WSX1, and CNTFR, as well as interaction with the formyl peptide receptor 2 (formylpeptide-like-1 receptor) have been published.[13][14]
Intracellular interaction with BAX, tBID, IGFBP3, and TRIM11 may also be required for the effects of humanin.[9][15][16][17]
Discovery
Humanin was the first mitochondria-derived peptide to be discovered.[3] Humanin was independently found by three different labs looking at different parameters. The first to publish, in 2001, was the Nishimoto lab, which found humanin while looking for possible proteins that could protect cells from amyloid beta, a major component of Alzheimer's disease.[5] The Reed lab found humanin when screening for proteins that could interact with Bcl-2-associated X protein (Bax), a major protein involved in apoptosis.[9] The Pinchas Cohen lab independently discovered humanin when screening for proteins that interact with IGFBP3.[15]
Research
Experiments using cultured cells have demonstrated that humanin has both neuroprotective as well as cytoprotective effects and experiments in rodents have found that it has protective effects in Alzheimer's disease models, Huntington's disease models and stroke models.[18]
Humanin is proposed to have myriad neuroprotective and cytoprotective effects. Both studies in cells and rodents have both found that administration of humanin or humanin derivatives increases survival and/or physiological parameters in Alzheimer's disease models.[19][20] In addition to Alzheimer's disease, humanin has other neuroprotective effects against models of Huntington's disease, prion disease, and stroke.[21][22][23]
Beyond the possible neuroprotective effects, humanin protects against oxidative stress, atherosclerotic plaque formation, and heart attack.[24][25][26][27] Humanin activates chaperone-mediated autophagy in a dose-dependent manner.[1] Humanin decreases production of inflammatory cytokines, which is part of its anti-apoptotic effect.[2] Metabolic effects have also been demonstrated and humanin helps improve survival of pancreatic beta-cells, which may help with type 1 diabetes,[28] and increases insulin sensitivity, which may help with type 2 diabetes.[29][4] In rats, the humanin analog appears to normalize glucose levels and reduce diabetes symptoms.[30]
Rattin shows the same ability as humanin to defend neurons from the toxicity of beta-amyloid, the cause of degeneration in Alzheimer's disease.[10]
Small humanin-like peptides are a group of peptides found in the mitochondrial 16S rRNA, and also possess retrograde signaling functions.
References
- ↑ 1.0 1.1 "Protective Mechanism of Humanin Against Oxidative Stress in Aging-Related Cardiovascular Diseases". Frontiers in Endocrinology 12: 683151. 2021. doi:10.3389/fendo.2021.683151. PMID 34177809.
- ↑ 2.0 2.1 "Mitochondria, immunosenescence and inflammaging: a role for mitokines?". Seminars in Immunopathology 42 (5): 607–617. 2020. doi:10.1007/s00281-020-00813-0. PMID 32757036.
- ↑ 3.0 3.1 3.2 3.3 "Mitochondria-derived peptides in aging and healthspan". Journal of Clinical Investigation 132 (9): e158449. 2022. doi:10.1172/JCI158449. PMID 35499074.
- ↑ 4.0 4.1 "Mitochondrial stress and mitokines in aging". Aging Cell 22 (2): e13770. 2023. doi:10.1111/acel.13770. PMID 36642986.
- ↑ 5.0 5.1 "A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and Abeta". Proceedings of the National Academy of Sciences of the United States of America 98 (11): 6336–41. May 2001. doi:10.1073/pnas.101133498. PMID 11371646. Bibcode: 2001PNAS...98.6336H.
- ↑ "Evidence for potential functionality of nuclearly-encoded humanin isoforms". Genomics 94 (4): 247–56. October 2009. doi:10.1016/j.ygeno.2009.05.006. PMID 19477263.
- ↑ 7.0 7.1 "Solution structure of humanin, a peptide against Alzheimer's disease-related neurotoxicity". Biochemical and Biophysical Research Communications 329 (1): 152–60. April 2005. doi:10.1016/j.bbrc.2005.01.100. PMID 15721287. http://www.rcsb.org/pdb/explore/remediatedSequence.do?structureId=1Y32. Retrieved 2014-07-07.
- ↑ 8.0 8.1 8.2 "The emerging role of the mitochondrial-derived peptide humanin in stress resistance". Journal of Molecular Endocrinology 50 (1): R11-9. February 2013. Feb 2013. doi:10.1530/JME-12-0203. PMID 23239898.
- ↑ 9.0 9.1 9.2 "Humanin peptide suppresses apoptosis by interfering with Bax activation". Nature 423 (6938): 456–61. May 2003. doi:10.1038/nature01627. PMID 12732850. Bibcode: 2003Natur.423..456G.
- ↑ 10.0 10.1 10.2 10.3 10.4 "A novel rat gene encoding a Humanin-like peptide endowed with broad neuroprotective activity". FASEB Journal 16 (10): 1331–3. August 2002. doi:10.1096/fj.02-0018fje. PMID 12154011.
- ↑ Kim, J; Choi, JW; Namkung, J (31 January 2021). "Expression Profile of Mouse Gm20594, Nuclear-Encoded Humanin-Like Gene.". Journal of Lifestyle Medicine 11 (1): 13–22. doi:10.15280/jlm.2021.11.1.13. PMID 33763338.
- ↑ "Mitochondrially derived peptides as novel regulators of metabolism". The Journal of Physiology 595 (21): 6613–6621. November 2017. doi:10.1113/JP274472. PMID 28574175.
- ↑ "Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor alpha/WSX-1/gp130". Molecular Biology of the Cell 20 (12): 2864–73. June 2009. doi:10.1091/mbc.E09-02-0168. PMID 19386761.
- ↑ "Humanin, a newly identified neuroprotective factor, uses the G protein-coupled formylpeptide receptor-like-1 as a functional receptor". Journal of Immunology 172 (11): 7078–85. June 2004. doi:10.4049/jimmunol.172.11.7078. PMID 15153530.
- ↑ 15.0 15.1 "Interaction between the Alzheimer's survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis". Proceedings of the National Academy of Sciences of the United States of America 100 (22): 13042–7. October 2003. doi:10.1073/pnas.2135111100. PMID 14561895. Bibcode: 2003PNAS..10013042I.
- ↑ "Humanin binds and nullifies Bid activity by blocking its activation of Bax and Bak". The Journal of Biological Chemistry 280 (16): 15815–24. April 2005. doi:10.1074/jbc.M411902200. PMID 15661737.
- ↑ "A tripartite motif protein TRIM11 binds and destabilizes Humanin, a neuroprotective peptide against Alzheimer's disease-relevant insults". The European Journal of Neuroscience 17 (6): 1150–8. March 2003. doi:10.1046/j.1460-9568.2003.02553.x. PMID 12670303.
- ↑ "The emerging role of the mitochondrial-derived peptide humanin in stress resistance". Journal of Molecular Endocrinology 50 (1): R11-9. February 2013. doi:10.1530/JME-12-0203. PMID 23239898.
- ↑ "A humanin derivative, S14G-HN, prevents amyloid-beta-induced memory impairment in mice". Journal of Neuroscience Research 79 (5): 714–23. March 2005. doi:10.1002/jnr.20391. PMID 15678515.
- ↑ "Detailed characterization of neuroprotection by a rescue factor humanin against various Alzheimer's disease-relevant insults". The Journal of Neuroscience 21 (23): 9235–45. December 2001. doi:10.1523/JNEUROSCI.21-23-09235.2001. PMID 11717357.
- ↑ "Humanin attenuates apoptosis induced by DRPLA proteins with expanded polyglutamine stretches". Journal of Molecular Neuroscience 25 (2): 165–9. 2005. doi:10.1385/JMN:25:2:165. PMID 15784964.
- ↑ "Humanin rescues cortical neurons from prion-peptide-induced apoptosis". Molecular and Cellular Neurosciences 25 (1): 95–102. January 2004. doi:10.1016/j.mcn.2003.09.017. PMID 14962743.
- ↑ "Humanin is a novel neuroprotective agent against stroke". Stroke 37 (10): 2613–9. October 2006. doi:10.1161/01.STR.0000242772.94277.1f. PMID 16960089.
- ↑ "Humanin is expressed in human vascular walls and has a cytoprotective effect against oxidized LDL-induced oxidative stress". Cardiovascular Research 88 (2): 360–6. November 2010. doi:10.1093/cvr/cvq191. PMID 20562421.
- ↑ "Humanin preserves endothelial function and prevents atherosclerotic plaque progression in hypercholesterolemic ApoE deficient mice". Atherosclerosis 219 (1): 65–73. November 2011. doi:10.1016/j.atherosclerosis.2011.06.038. PMID 21763658.
- ↑ Westermark, Per, ed (2012). "Humanin, a cytoprotective peptide, is expressed in carotid atherosclerotic [corrected plaques in humans"]. PLOS ONE 7 (2): e31065. doi:10.1371/journal.pone.0031065. PMID 22328926. Bibcode: 2012PLoSO...731065Z.
- ↑ "Acute humanin therapy attenuates myocardial ischemia and reperfusion injury in mice". Arteriosclerosis, Thrombosis, and Vascular Biology 30 (10): 1940–8. October 2010. doi:10.1161/ATVBAHA.110.205997. PMID 20651283.
- ↑ "The neurosurvival factor Humanin inhibits beta-cell apoptosis via signal transducer and activator of transcription 3 activation and delays and ameliorates diabetes in nonobese diabetic mice". Metabolism 59 (3): 343–9. March 2010. doi:10.1016/j.metabol.2009.08.001. PMID 19800083.
- ↑ Vella, Adrian, ed (July 2009). "Humanin: a novel central regulator of peripheral insulin action". PLOS ONE 4 (7): e6334. doi:10.1371/journal.pone.0006334. PMID 19623253. Bibcode: 2009PLoSO...4.6334M.
- ↑ Hall, Stephen S. (March 2012). "New Clues to a Long Life". National Geographic. http://ngm.nationalgeographic.com/2013/05/longevity/hall-text.
 |
