Chemistry:Hydrazinium azide
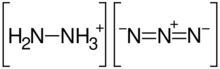
| |
| Names | |
|---|---|
| IUPAC name
Hydrazinium azide
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| H5N5 | |
| Molar mass | 75.075 g·mol−1 |
| Appearance | White solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hydrazinium azide or hydrazine azide is a chemical compound with formula H5N5 or [N2H+5][N−3]. It is a salt of the hydrazinium cation N2H+5 and the azide anion N−3. It can be seen as a derivative of hydrazine N2H4 and hydrazoic acid HN3. It is an unstable solid.
The compound is of scientific interest because of its high nitrogen content (93% nitrogen by weight) and explosive properties.[1]
Structure
The solid undergoes structural phase transition to a different crystalline arrangement at a pressure of 13 GPa.[2]
Chemistry
Hydrazinium azide decomposes explosively into hydrazine, ammonia, and nitrogen gas:[3]
- 12 N5H5 → 3 N2H4 + 16 NH3 + 19 N2
Crystallization with an equimolar amount hydrazine yields the solid hydrazinium azide hydrazinate, [N2H+5][N−3]·[N2H4], or N7H9, as monoclinic crystals. This compound is less hygroscopic and less volatile than pure hydrazinium azide. It decomposes explosively into nitrogen, hydrogen, and ammonia.[4]
At pressure of 40 GPa, hydrazinium azide decomposes yielding a linear nitrogen allotrope N8 or N≡−−N=N−−≡N, that decomposes to ε-N2 below 25 GPa.[2]
Reaction of hydrazinium azide with sulfuric acid gives quantitative yields of pure hydrazinediium sulfate and hydrazoic acid:[5]
- [N2H+5][N−3] + H2SO4 → [N2H2+6][SO2−4] + HN3
See also
- Ammonium azide, [NH+4][N−3]
References
- ↑ Chiglien, G.; Etienne, J.; Jaulmes, S.; Laruelle, P. (15 September 1974). "Structure cristalline de l'azoture d'hydrazinium, N5H5". Acta Crystallographica Section B 30 (9): 2229–2233. doi:10.1107/S0567740874006790. Bibcode: 1974AcCrB..30.2229C.
- ↑ 2.0 2.1 Duwal, Sakun; Ryu, Young-Jay; Kim, Minseob; Yoo, Choong-Shik; Bang, Sora; Kim, Kyungtae; Hur, Nam Hwi (7 April 2018). "Transformation of hydrazinium azide to molecular N8 at 40 GPa". The Journal of Chemical Physics 148 (13): 134310. doi:10.1063/1.5021976. PMID 29626901. Bibcode: 2018JChPh.148m4310D.
- ↑ G. B. Manelis (2003). Thermal decomposition and combustion of explosives and propellants. CRC Press. p. 235. ISBN 0-415-29984-5. https://books.google.com/books?id=_Xz7_o23J_0C&pg=PA235.
- ↑ Hammerl, Anton; Klapötke, Thomas M.; Piotrowski, Holger; Holl, Gerhard; Kaiser, Manfred (2001). "Synthesis and Characterization of Hydrazinium Azide Hydrazinate". Propellants, Explosives, Pyrotechnics 26 (4): 161–164. doi:10.1002/1521-4087(200110)26:4<161::AID-PREP161>3.0.CO;2-O.
- ↑ Klapötke, T.; Peter S. White; Inis C. Tornieporth-Oetting (1996). "Reaction of hydrazinium azide with sulfuric acid: the X-ray structure of [N2H6][SO4]". Polyhedron 15 (15): 2579–2582. doi:10.1016/0277-5387(95)00527-7.
Further reading
- Schmidt, Eckart W. (2022). "Hydrazinium Salt Oxidizers". Encyclopedia of Oxidizers. De Gruyter. pp. 1129–1139. doi:10.1515/9783110750294-009. ISBN 978-3-11-075029-4.
 |
