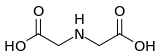
Chemistry:Iminodiacetic acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
2,2′-Azanediyldiacetic acid | |
| Other names
2-(Carboxymethylamino)acetic acid
Diglycolamidic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| 878499 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
| MeSH | imnodiacetic+acid |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C4H7NO4 | |
| Molar mass | 133.103 g·mol−1 |
| Appearance | Colourless crystals |
| Density | 1.436 g mL−1 |
| log P | 1.84 |
| Acidity (pKa) | 1.873 |
| Basicity (pKb) | 12.124 |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
−933.9–−931.3 kJ mol−1 |
Std enthalpy of
combustion (ΔcH⦵298) |
−1.6430–−1.6406 MJ mol−1 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | WARNING |
| H315, H319, H335 | |
| P261, P305+351+338 | |
| Flash point | 178 °C (352 °F; 451 K) |
| Related compounds | |
Related alkanoic acids
|
|
Related compounds
|
N-Acetylglycinamide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Iminodiacetic acid is the organic compound with the formula HN(CH2CO2H)2, often abbreviated to IDA. A white solid, the compound is a dicarboxylic acid amine (the nitrogen atom forms a secondary amino group, not an imino group as the name suggests). The iminodiacetate dianion is a tridentate ligand, forming metal complexes by forming two, fused, five membered chelate rings.[1] The proton on the nitrogen atom can be replaced by a carbon atom of a polymer to create an ion-exchange resin, such as chelex 100. Complexes of IDA and EDTA were introduced in the early 1950's by Schwarzenbach.[2]
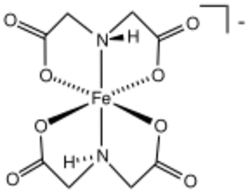
IDA forms stronger complexes than the bidentate ligand glycine and weaker complexes than the tetradentate ligand nitrilotriacetic acid. It can also act as a bidentate ligand through its two carboxylate groups. Several technetium-99m complexes are used in cholescintigraphy scans (also known as hepatobiliary iminodiacetic acid scans) to evaluate the health and function of the gallbladder.[3][4]
Iminodiacetic acid is an important intermediate in one of the two main industrial processes used to manufacture the herbicide glyphosate. It is used in capillary electrophoresis for modulating peptide mobility. It is also used as a precursor for the manufacture of the indicator xylenol orange.
Related compounds
- N-Methylimidodiacetic acid (MIDA)
- N-(2-Carboxyethyl)iminodiacetic acid
- Nitrilotriacetic acid (NTA)
- N-hydroxyiminodiacetic acid (HIDA), HON(CH
2CO
2H)
2 (registry number = 87339-38-6)[5] See HIDA scan.
References
- ↑ 1.0 1.1 Schmitt, Wolfgang; Jordan, Peter A.; Henderson, Richard K.; Moore, Geoffrey R.; Anson, Christopher E.; Powell, Annie K. (2002). "Synthesis, structures and properties of hydrolytic Al(III) aggregates and Fe(III) analogues formed with iminodiacetate-based chelating ligands". Coordination Chemistry Reviews 228 (2): 115–126. doi:10.1016/S0010-8545(02)00110-8.
- ↑ Schwarzenbach, G (1952). "Der Chelateffekt". Helv. Chim. Acta 35 (7): 2344–2359. doi:10.1002/hlca.19520350721.
- ↑ Michael, Picco. "HIDA scan (cholescintigraphy): Why is it performed?". Mayo Clinic. http://www.mayoclinic.com/health/hida-scan/AN00424.
- ↑ Krishnamurthy, Gerbail T.; Krishnamurthy, Shakuntala (2009). "Imaging Agents". Nuclear Hepatology: A Textbook of Hepatobiliary Diseases. Springer. pp. 54–57. ISBN 978-3-642-00647-0. https://books.google.com/books?id=IXaPDIiGeg4C&q=iminodiacetic+acid. Retrieved 19 December 2015.
- ↑ Hubregtse, Ton; Hanefeld, Ulf; Arends, Isabel W. C. E. (2007). "Stabilizing Factors for Vanadium(IV) in Amavadin". European Journal of Organic Chemistry 2007 (15): 2413–2422. doi:10.1002/ejoc.200601053.
 |

