Chemistry:Introduction to the heaviest elements
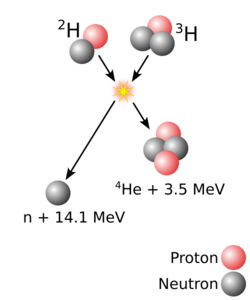
The heaviest[lower-alpha 1] atomic nuclei are created in nuclear reactions that combine two other nuclei of unequal size[lower-alpha 2] into one; roughly, the more unequal the two nuclei in terms of mass, the greater the possibility that the two react.[7] The material made of the heavier nuclei is made into a target, which is then bombarded by the beam of lighter nuclei. Two nuclei can fuse into one only if they approach each other closely enough; normally, nuclei (all positively charged) repel each other due to electrostatic repulsion. The strong interaction can overcome this repulsion but only within a very short distance from a nucleus; beam nuclei are thus greatly accelerated in order to make such repulsion insignificant compared to the velocity of the beam nucleus.[8] Coming close alone is not enough for two nuclei to fuse: when two nuclei approach each other, they usually remain together for approximately 10−20 seconds and then part ways (not necessarily in the same composition as before the reaction) rather than form a single nucleus.[8][9] If fusion does occur, the temporary merger—termed a compound nucleus—is an excited state. To lose its excitation energy and reach a more stable state, a compound nucleus either fissions or ejects one or several neutrons,[lower-alpha 3] which carry away the energy. This occurs in approximately 10−16 seconds after the initial collision.[10][lower-alpha 4]
The beam passes through the target and reaches the next chamber, the separator; if a new nucleus is produced, it is carried with this beam.[13] In the separator, the newly produced nucleus is separated from other nuclides (that of the original beam and any other reaction products)[lower-alpha 5] and transferred to a surface-barrier detector, which stops the nucleus. The exact location of the upcoming impact on the detector is marked; also marked are its energy and the time of the arrival.[13] The transfer takes about 10−6 seconds; in order to be detected, the nucleus must survive this long.[16] The nucleus is recorded again once its decay is registered, and the location, the energy, and the time of the decay are measured.[13]
Stability of a nucleus is provided by the strong interaction. However, its range is very short; as nuclei become larger, their influence on the outermost nucleons (protons and neutrons) weakens. At the same time, the nucleus is torn apart by electrostatic repulsion between protons, as it has unlimited range.[17] Nuclei of the heaviest elements are thus theoretically predicted[18] and have so far been observed[19] to primarily decay via decay modes that are caused by such repulsion: alpha decay and spontaneous fission;[lower-alpha 6] these modes are predominant for nuclei of superheavy elements. Alpha decays are registered by the emitted alpha particles, and the decay products are easy to determine before the actual decay; if such a decay or a series of consecutive decays produces a known nucleus, the original product of a reaction can be determined arithmetically.[lower-alpha 7] Spontaneous fission, however, produces various nuclei as products, so the original nuclide cannot be determined from its daughters.[lower-alpha 8]
The information available to physicists aiming to synthesize one of the heaviest elements is thus the information collected at the detectors: location, energy, and time of arrival of a particle to the detector, and those of its decay. The physicists analyze this data and seek to conclude that it was indeed caused by a new element and could not have been caused by a different nuclide than the one claimed. Often, provided data is insufficient for a conclusion that a new element was definitely created and there is no other explanation for the observed effects; errors in interpreting data have been made.[lower-alpha 9]
Notes
- ↑ In nuclear physics, an element is called heavy if its atomic number is high; lead (element 82) is one example of such a heavy element. The term "superheavy elements" typically refers to elements with atomic number greater than 103 (although there are other definitions, such as atomic number greater than 100[2] or 112;[3] sometimes, the term is presented an equivalent to the term "transactinide", which puts an upper limit before the beginning of the hypothetical superactinide series).[4] Terms "heavy isotopes" (of a given element) and "heavy nuclei" mean what could be understood in the common language—isotopes of high mass (for the given element) and nuclei of high mass, respectively.
- ↑ In 2009, a team at JINR led by Oganessian published results of their attempt to create hassium in a symmetric 136Xe + 136Xe reaction. They failed to observe a single atom in such a reaction, putting the upper limit on the cross section, the measure of probability of a nuclear reaction, as 2.5 pb.[5] In comparison, the reaction that resulted in hassium discovery, 208Pb + 58Fe, had a cross section of ~20 pb (more specifically, 19+19−11 pb), as estimated by the discoverers.[6]
- ↑ The greater the excitation energy, the more neutrons are ejected. If the excitation energy is lower than energy binding each neutron to the rest of the nucleus, neutrons are not emitted; instead, the compound nucleus de-excites by emitting a gamma ray.[10]
- ↑ The definition by the IUPAC/IUPAP Joint Working Party states that a chemical element can only be recognized as discovered if a nucleus of it has not decayed within 10−14 seconds. This value was chosen as an estimate of how long it takes a nucleus to acquire its outer electrons and thus display its chemical properties.[11] This figure also marks the generally accepted upper limit for lifetime of a compound nucleus.[12]
- ↑ This separation is based on that the resulting nuclei move past the target more slowly then the unreacted beam nuclei. The separator contains electric and magnetic fields whose effects on a moving particle cancel out for a specific velocity of a particle.[14] Such separation can also be aided by a time-of-flight measurement and a recoil energy measurement; a combination of the two may allow to estimate the mass of a nucleus.[15]
- ↑ Not all decay modes are caused by electrostatic repulsion. For example, beta decay is caused by the weak interaction.[20]
- ↑ Since mass of a nucleus is not measured directly but is rather calculated from that of another nucleus, such measurement is called indirect. Direct measurements are also possible, but for the most part they have remained unavailable for heaviest nuclei.[21] The first direct measurement of mass of a superheavy nucleus was reported in 2018 at LBNL.[22] Mass was determined from the location of a nucleus after the transfer (the location helps determine its trajectory, which is linked to the mass-to-charge ratio of the nucleus, since the transfer was done in presence of a magnet).[23]
- ↑ Spontaneous fission was discovered by Soviet physicist Georgy Flerov,[24] a leading scientist at JINR, and thus it was a "hobbyhorse" for the facility.[25] In contrast, the LBL scientists believed fission information was not sufficient for a claim of synthesis of an element. They believed spontaneous fission had not been studied enough to use it for identification of a new element, since there was a difficulty of establishing that a compound nucleus had only ejected neutrons and not charged particles like protons or alpha particles.[12] They thus preferred to link new isotopes to the already known ones by successive alpha decays.[24]
- ↑ For instance, element 102 was mistakenly identified in 1957 at the Nobel Institute of Physics in Stockholm, Stockholm County, Sweden.[26] There were no earlier definitive claims of creation of this element, and the element was assigned a name by its Swedish, American, and British discoverers, nobelium. It was later shown that the identification was incorrect.[27] The following year, LBNL was unable to reproduce the Swedish results and announced instead their synthesis of the element; that claim was also disproved later.[27] JINR insisted that they were the first to create the element and suggested a name of their own for the new element, joliotium;[28] the Soviet name was also not accepted (JINR later referred to the naming of element 102 as "hasty").[29] The name "nobelium" remained unchanged on account of its widespread usage.[30]
References
- ↑ Wakhle, A.; Simenel, C.; Hinde, D. J. et al. (2015). Simenel, C.; Gomes, P. R. S.; Hinde, D. J. et al.. eds. "Comparing Experimental and Theoretical Quasifission Mass Angle Distributions". European Physical Journal Web of Conferences 86: 00061. doi:10.1051/epjconf/20158600061. ISSN 2100-014X. Bibcode: 2015EPJWC..8600061W.
- ↑ Krämer, K. (2016). "Explainer: superheavy elements". https://www.chemistryworld.com/news/explainer-superheavy-elements/1010345.article.
- ↑ "Discovery of Elements 113 and 115". Lawrence Livermore National Laboratory. https://pls.llnl.gov/research-and-development/nuclear-science/project-highlights/livermorium/elements-113-and-115.
- ↑ Eliav, E.; Kaldor, U.; Borschevsky, A. (2018). "Electronic Structure of the Transactinide Atoms". in Scott, R. A.. Encyclopedia of Inorganic and Bioinorganic Chemistry. John Wiley & Sons. pp. 1–16. doi:10.1002/9781119951438.eibc2632. ISBN 978-1-119-95143-8.
- ↑ Oganessian, Yu. Ts.; Dmitriev, S. N.; Yeremin, A. V. et al. (2009). "Attempt to produce the isotopes of element 108 in the fusion reaction 136Xe + 136Xe". Physical Review C 79 (2): 024608. doi:10.1103/PhysRevC.79.024608. ISSN 0556-2813.
- ↑ Münzenberg, G.; Armbruster, P.; Folger, H. et al. (1984). "The identification of element 108". Zeitschrift für Physik A 317 (2): 235–236. doi:10.1007/BF01421260. Bibcode: 1984ZPhyA.317..235M. http://www.gsi-heavy-ion-researchcenter.org/forschung/kp/kp2/ship/108-discovery.pdf. Retrieved 20 October 2012.
- ↑ Subramanian, S. (2019). "Making New Elements Doesn't Pay. Just Ask This Berkeley Scientist". https://www.bloomberg.com/news/features/2019-08-28/making-new-elements-doesn-t-pay-just-ask-this-berkeley-scientist.
- ↑ 8.0 8.1 Ivanov, D. (2019). "Сверхтяжелые шаги в неизвестное" (in ru). https://nplus1.ru/material/2019/03/25/120-element.
- ↑ Hinde, D. (2014). "Something new and superheavy at the periodic table". http://theconversation.com/something-new-and-superheavy-at-the-periodic-table-26286.
- ↑ 10.0 10.1 Krása, A. (2010). "Neutron Sources for ADS". Czech Technical University in Prague. pp. 4–8. http://pdfs.semanticscholar.org/ba08/30dcab221b45ca5bcc3cfa8ae82558d624e7.pdf.
- ↑ Wapstra, A. H. (1991). "Criteria that must be satisfied for the discovery of a new chemical element to be recognized". Pure and Applied Chemistry 63 (6): 883. doi:10.1351/pac199163060879. ISSN 1365-3075. http://publications.iupac.org/pac/pdf/1991/pdf/6306x0879.pdf. Retrieved 2020-08-28.
- ↑ 12.0 12.1 Hyde, E. K.; Hoffman, D. C.; Keller, O. L. (1987). "A History and Analysis of the Discovery of Elements 104 and 105". Radiochimica Acta 42 (2): 67–68. doi:10.1524/ract.1987.42.2.57. ISSN 2193-3405. http://www.escholarship.org/uc/item/05x8w9h7.
- ↑ 13.0 13.1 13.2 Chemistry World (2016). "How to Make Superheavy Elements and Finish the Periodic Table [Video"]. https://www.scientificamerican.com/article/how-to-make-superheavy-elements-and-finish-the-periodic-table-video/.
- ↑ Hoffman, Ghiorso & Seaborg 2000, p. 334.
- ↑ Hoffman, Ghiorso & Seaborg 2000, p. 335.
- ↑ Zagrebaev, Karpov & Greiner 2013, p. 3.
- ↑ Beiser 2003, p. 432.
- ↑ Staszczak, A.; Baran, A.; Nazarewicz, W. (2013). "Spontaneous fission modes and lifetimes of superheavy elements in the nuclear density functional theory". Physical Review C 87 (2): 024320–1. doi:10.1103/physrevc.87.024320. ISSN 0556-2813. Bibcode: 2013PhRvC..87b4320S.
- ↑ Audi, G.; Kondev, F. G.; Wang, M. et al. (2017). "The NUBASE2016 evaluation of nuclear properties". Chinese Physics C 41 (3): 030001-128–030001-138. doi:10.1088/1674-1137/41/3/030001. Bibcode: 2017ChPhC..41c0001A.
- ↑ Beiser 2003, p. 439.
- ↑ Oganessian, Yu. Ts.; Rykaczewski, K. P. (2015). "A beachhead on the island of stability". Physics Today 68 (8): 32–38. doi:10.1063/PT.3.2880. ISSN 0031-9228. Bibcode: 2015PhT....68h..32O. https://www.osti.gov/biblio/1337838.
- ↑ Grant, A. (2018). "Weighing the heaviest elements". Physics Today. doi:10.1063/PT.6.1.20181113a.
- ↑ Howes, L. (2019). "Exploring the superheavy elements at the end of the periodic table". https://cen.acs.org/physical-chemistry/periodic-table/IYPT-Exploring-the-superheavy-elements-at-the-end-of-the-periodic-table/97/i21.
- ↑ 24.0 24.1 Robinson, A. E. (2019). "The Transfermium Wars: Scientific Brawling and Name-Calling during the Cold War". Distillations. https://www.sciencehistory.org/distillations/the-transfermium-wars-scientific-brawling-and-name-calling-during-the-cold-war. Retrieved 2020-02-22.
- ↑ "Популярная библиотека химических элементов. Сиборгий (экавольфрам)" (in ru). http://n-t.ru/ri/ps/pb106.htm. Reprinted from "Экавольфрам" (in ru). Популярная библиотека химических элементов. Серебро — Нильсборий и далее. Nauka. 1977.
- ↑ "Nobelium – Element information, properties and uses | Periodic Table". Royal Society of Chemistry. https://www.rsc.org/periodic-table/element/102/nobelium.
- ↑ 27.0 27.1 Kragh 2018, pp. 38–39.
- ↑ Kragh 2018, p. 40.
- ↑ Ghiorso, A.; Seaborg, G. T.; Oganessian, Yu. Ts. et al. (1993). "Responses on the report 'Discovery of the Transfermium elements' followed by reply to the responses by Transfermium Working Group". Pure and Applied Chemistry 65 (8): 1815–1824. doi:10.1351/pac199365081815. https://www.iupac.org/publications/pac/1993/pdf/6508x1815.pdf. Retrieved 7 September 2016.
- ↑ Commission on Nomenclature of Inorganic Chemistry (1997). "Names and symbols of transfermium elements (IUPAC Recommendations 1997)". Pure and Applied Chemistry 69 (12): 2471–2474. doi:10.1351/pac199769122471. http://publications.iupac.org/pac/pdf/1997/pdf/6912x2471.pdf.
Bibliography
- Audi, G.; Kondev, F. G.; Wang, M. et al. (2017). "The NUBASE2016 evaluation of nuclear properties". Chinese Physics C 41 (3): 030001. doi:10.1088/1674-1137/41/3/030001. Bibcode: 2017ChPhC..41c0001A.
pp. 030001-1–030001-17, pp. 030001-18–030001-138, Table I. The NUBASE2016 table of nuclear and decay properties - Beiser, A. (2003). Concepts of modern physics (6th ed.). McGraw-Hill. ISBN 978-0-07-244848-1. OCLC 48965418.
- Hoffman, D. C.; Ghiorso, A.; Seaborg, G. T. (2000). The Transuranium People: The Inside Story. World Scientific. ISBN 978-1-78-326244-1.
- Kragh, H. (2018). From Transuranic to Superheavy Elements: A Story of Dispute and Creation. Springer. ISBN 978-3-319-75813-8.
- Zagrebaev, V.; Karpov, A.; Greiner, W. (2013). "Future of superheavy element research: Which nuclei could be synthesized within the next few years?". Journal of Physics 420 (1): 012001. doi:10.1088/1742-6596/420/1/012001. ISSN 1742-6588. Bibcode: 2013JPhCS.420a2001Z.
