Chemistry:Isorhapontin
From HandWiki
| |
| Names | |
|---|---|
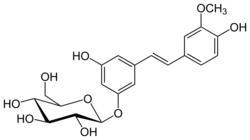
| IUPAC name
3-Hydroxy-5-[(1E)-2-(4-hydroxy-3-methoxyphenyl)ethen-1-yl]phenoxy β-D-glucopyranoside
| |
| Systematic IUPAC name
(2S,3R,4S,5S,6R)-2-{3-Hydroxy-5-[(1E)-2-(4-hydroxy-3-methoxyphenyl)ethen-1-yl]phenoxy}-6-(hydroxymethyl)oxane-3,4,5-triol | |
| Other names
Isorhapontigenin glucoside
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C21H24O9 | |
| Molar mass | 420.41 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Isorhapontin is a stilbenoid. It is the glucoside of isorhapontigenin. It can be found in mycorrhizal and non-mycorrhizal roots of Norway spruces (Picea abies),[1] in the bark of Picea sitchensis[2][3] or in white spruce (Picea glauca).[4]
References
- ↑ Münzenberger, B; Heilemann, J; Strack, D; Kottke, I; Oberwinkler, F (1990). "Phenolics of mycorrhizas and non-mycorrhizal roots of Norway spruce". Planta 182 (1): 142–148. doi:10.1007/BF00239996. PMID 24197010.
- ↑ Stilbene glucosides in the bark of Picea sitchensis. Masakazu Aritomi, Dervilla M.X. Donnelly, Phytochemistry, Volume 15, Issue 12, 1976, Pages 2006–2008, doi:10.1016/S0031-9422(00)88881-0
- ↑ Astringin and isorhapontin distribution in Sitka spruce trees. Claudia D. Toscano Underwood and Raymond B. Pearce, Phytochemistry, Volume 30, Issue 7, 1991, Pages 2183–2189, doi:10.1016/0031-9422(91)83610-W
- ↑ Hammerbacher, A.; Ralph, S. G.; Bohlmann, J.; Fenning, T. M.; Gershenzon, J.; Schmidt, A. (2011). "Biosynthesis of the Major Tetrahydroxystilbenes in Spruce, Astringin and Isorhapontin, Proceeds via Resveratrol and is Enhanced by Fungal Infection". Plant Physiology 157 (2): 876–890. doi:10.1104/pp.111.181420. PMID 21865488.
 |
