Chemistry:Pinosylvin
| |
| Names | |
|---|---|
| Preferred IUPAC name
5-[(1E)-2-Phenylethen-1-yl]benzene-1,3-diol | |
| Other names
(E)-3,5-Stilbenediol
trans-3,5-Dihydroxystilbene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C14H12O2 | |
| Molar mass | 212.248 g·mol−1 |
| Appearance | white solid |
| Melting point | 153 to 155 °C (307 to 311 °F; 426 to 428 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
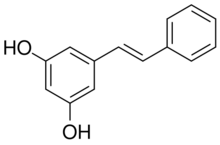
Pinosylvin is an organic compound with the formula C6H5CH=CHC6H3(OH)2. A white solid, it is related to trans-stilbene, but with two hydroxy groups on one of the phenyl substituents. It is very soluble in many organic solvents, such as acetone.[1]
Occurrence
Pinosylvin is produced in plants in response to fungal infections, ozone-induced stress, and physical damage for example.[2] It is a fungitoxin protecting the wood from fungal infection.[3] It is present in the heartwood of Pinaceae[2] and also found in Gnetum cleistostachyum.[4]
Injected in rats, pinosylvin undergoes rapid glucuronidation and a poor bioavailability.[5]
Biosynthesis
Pinosylvin synthase, an enzyme, catalyzes the biosynthesis of pinosylvin from malonyl-CoA and cinnamoyl-CoA:
- 3 malonyl-S-CoA + cinnamoyl-S-CoA → 4 CoA-SH + pinosylvin + 4 CO2
This biosynthesis is noteworthy because plant biosyntheses employing cinnamic acid as a starting point are rare compared to the more common use of p-coumaric acid. Two other compounds produced from cinnamic acid are anigorufone and curcumin.[6][7]
References
- ↑ M., Haynes, William (2014). "3". CRC Handbook of Chemistry and Physics, 95th Edition (95th ed.). Hoboken: CRC Press. pp. 458. ISBN 9781482208689. OCLC 908078665.
- ↑ 2.0 2.1 Hovelstad, Hanne; Leirset, Ingebjorg; Oyaas, Karin; Fiksdahl, Anne (2006-01-31). "Screening analyses of pinosylvin stilbenes, resin acids and lignans in Norwegian conifers". Molecules (Basel, Switzerland) 11 (1): 103–114. doi:10.3390/11010103. ISSN 1420-3049. PMID 17962750.
- ↑ Lee, S. K.; Lee, H. J.; Min, H. Y.; Park, E. J.; Lee, K. M.; Ahn, Y. H.; Cho, Y. J.; Pyee, J. H. (March 2005). "Antibacterial and antifungal activity of pinosylvin, a constituent of pine". Fitoterapia 76 (2): 258–260. doi:10.1016/j.fitote.2004.12.004. ISSN 0367-326X. PMID 15752644.
- ↑ Yao, Chun-Suo; Lin, Mao; Liu, Xin; Wang, Ying-Hong (April 2005). "Stilbene derivatives from Gnetum cleistostachyum". Journal of Asian Natural Products Research 7 (2): 131–137. doi:10.1080/10286020310001625102. ISSN 1028-6020. PMID 15621615.
- ↑ Roupe, Kathryn A.; Yáñez, Jaime A.; Teng, Xiao Wei; Davies, Neal M. (November 2006). "Pharmacokinetics of selected stilbenes: rhapontigenin, piceatannol and pinosylvin in rats". The Journal of Pharmacy and Pharmacology 58 (11): 1443–1450. doi:10.1211/jpp.58.11.0004. ISSN 0022-3573. PMID 17132206.
- ↑ Schmitt, B.; Hölscher, D.; Schneider, B. (February 2000). "Variability of Phenylpropanoid PBiosynthesis of Phenylphenalenones in Anigozanthos preissii". Phytochemistry 53 (3): 331–337. doi:10.1016/s0031-9422(99)00544-0. ISSN 0031-9422. PMID 10703053.
- ↑ Kita, Tomoko; Imai, Shinsuke; Sawada, Hiroshi; Kumagai, Hidehiko; Seto, Haruo (July 2008). "The Biosynthetic Pathway of Curcuminoid in Turmeric (Curcuma longa) as Revealed by 13C-Labeled Precursors". Bioscience, Biotechnology, and Biochemistry 72 (7): 1789–1798. doi:10.1271/bbb.80075. ISSN 1347-6947. PMID 18603793.
 |

