Chemistry:Resveratrol
| |
| |
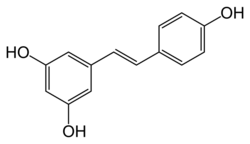
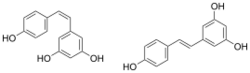 Chemical structures of cis- ((Z)-resveratrol, left) and trans-resveratrol ((E)-resveratrol, right)[1]
| |
| Names | |
|---|---|
| Preferred IUPAC name
5-[(E)-2-(4-Hydroxyphenyl)ethen-1-yl]benzene-1,3-diol | |
| Other names
trans-3,5,4′-Trihydroxystilbene;
3,4′,5-Stilbenetriol; trans-Resveratrol; (E)-5-(p-Hydroxystyryl)resorcinol; (E)-5-(4-hydroxystyryl)benzene-1,3-diol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C14H12O3 | |
| Molar mass | 228.247 g·mol−1 |
| Appearance | white powder with slight yellow cast |
| Melting point | 261 to 263 °C (502 to 505 °F; 534 to 536 K)[2] |
| Solubility in water | 0.03 g/L |
| Solubility in DMSO | 16 g/L |
| Solubility in ethanol | 50 g/L |
| UV-vis (λmax) | 304nm (trans-resveratrol, in water) 286nm (cis-resveratrol, in water)[1] |
| Hazards | |
| Safety data sheet | Fisher Scientific[2] Sigma Aldrich[3] |
| GHS pictograms | 
|
| GHS Signal word | warning |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
23.2 μM (5.29 g)[4] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a stilbenoid, a type of natural phenol, and a phytoalexin produced by several plants in response to injury or when the plant is under attack by pathogens, such as bacteria or fungi.[6][7] Sources of resveratrol in food include the skin of grapes, blueberries, raspberries, mulberries, and peanuts.[8][9]
Although commonly used as a dietary supplement and studied in laboratory models of human diseases,[10] there is no high-quality evidence that resveratrol improves lifespan or has a substantial effect on any human disease.[11][12]
Research
Resveratrol has been studied for its potential therapeutic use,[13] with little evidence of anti-disease effects or health benefits in humans.[6][10][14]
Cardiovascular disease
There is no evidence of benefit from resveratrol in people who already have heart disease.[10][15] A 2018 meta-analysis found no effect on systolic or diastolic blood pressure; a sub-analysis revealed a 2 mmHg decrease in systolic pressure only from resveratrol doses of 300 mg per day, and only in diabetic people.[16] A 2014 Chinese meta-analysis found no effect on systolic or diastolic blood pressure; a sub-analysis found an 11.90 mmHg reduction in systolic blood pressure from resveratrol doses of 150 mg per day.[17]
Cancer
As of 2020[update], there is no evidence of an effect of resveratrol on cancer in humans.[10][18]
Metabolic syndrome
There is no conclusive evidence for an effect of resveratrol on human metabolic syndrome.[10][19][20] One 2015 review found little evidence for use of resveratrol to treat diabetes.[21] A 2015 meta-analysis found little evidence for an effect of resveratrol on diabetes biomarkers.[22]
One review found limited evidence that resveratrol lowered fasting plasma glucose in people with diabetes.[23] Two reviews indicated that resveratrol supplementation may reduce body weight and body mass index, but not fat mass or total blood cholesterol.[24][25] A 2018 review found that resveratrol supplementation may reduce biomarkers of inflammation, TNF-α and C-reactive protein.[26]
Lifespan
There is insufficient evidence to indicate that consuming resveratrol has an effect on human lifespan.[11]
Cognition
Resveratrol has been assessed for a possible effect on cognition, but with mixed evidence for an effect. One review concluded that resveratrol had no effect on neurological function, but reported that supplementation improved recognition and mood, although there were inconsistencies in study designs and results.[27]
Diabetes
Although animal experiments have found some evidence that resveratrol may help improve insulin sensitivity and so potentially help manage diabetes, subsequent research on people is limited and does not support the use of resveratrol for this purpose.[28]
Other
There is no significant evidence that resveratrol affects vascular endothelial function, neuroinflammation, Alzheimer's disease, skin infections or aging skin.[6][10] A 2019 review of human studies found mixed effects of resveratrol on certain bone biomarkers, such as increases in blood and bone alkaline phosphatase, while reporting no effect on other biomarkers, such as calcium and collagen.[29]
Pharmacology
Pharmacodynamics
Resveratrol has been identified as a pan-assay interference compound, which produces positive results in many different laboratory assays.[30] Its ability for varied interactions may be due to direct effects on cell membranes.[31]
As of 2015, many specific biological targets for resveratrol had been identified, including NQO2 (alone and in interaction with AKT1), GSTP1, estrogen receptor beta, CBR1, and integrin αVβ. It was unclear at that time if any or all of these were responsible for the observed effects in cells and model organisms.[32]
Pharmacokinetics
The viability of an oral delivery method is unlikely due to the low aqueous solubility of the molecule. The bioavailability of resveratrol is about 0.5% due to extensive hepatic glucuronidation and sulfation.[33] Glucuronidation occurs in the intestine as well as in the liver, whereas sulfonation not only occurs in the liver but in the intestine and by microbial gut activity.[34] Due to rapid metabolism, the half-life of resveratrol is short (about 8–14 minutes), but the half-life of the sulphate and glucoronide metabolites is above 9 hours.[35]
Metabolism
Resveratrol is extensively metabolized in the body,[6] with the liver and intestines as the major sites of its metabolism.[36][35] Liver metabolites are products of phase II (conjugation) enzymes,[37] which are themselves induced by resveratrol in vitro.[38]
Chemistry
Resveratrol (3,5,4'-trihydroxystilbene) is a stilbenoid, a derivative of stilbene.[6] It exists as two geometric isomers: cis- (Z) and trans- (E), with the trans-isomer shown in the top image. Resveratrol exists conjugated to glucose.[39]
The trans- form can undergo photoisomerization to the cis- form when exposed to ultraviolet irradiation.[40][41]
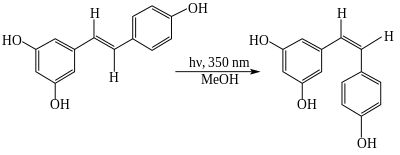
UV irradiation to cis-resveratrol induces further photochemical reaction, producing a fluorescent molecule named "Resveratrone".[42]
Trans-resveratrol in the powder form was found to be stable under "accelerated stability" conditions of 75% humidity and 40 °C in the presence of air.[43] The trans isomer is also stabilized by the presence of transport proteins.[44] Resveratrol content also was stable in the skins of grapes and pomace taken after fermentation and stored for a long period.[45] lH- and 13C-NMR data for the four most common forms of resveratrols are reported in literature.[39]
Biosynthesis
Resveratrol is produced in plants via the enzyme resveratrol synthase (stilbene synthase).[46][47] Its immediate precursor is a tetraketide derived from malonyl CoA and 4-coumaroyl CoA.[46][47] The latter is derived from phenylalanine.[48]
Biotransformation
The grapevine fungal pathogen Botrytis cinerea is able to oxidise resveratrol into metabolites showing attenuated antifungal activities. Those include the resveratrol dimers restrytisol A, B, and C, resveratrol trans-dehydrodimer, leachinol F, and pallidol.[49] The soil bacterium Bacillus cereus can be used to transform resveratrol into piceid (resveratrol 3-O-beta-D-glucoside).[50]
Adverse effects
Only a few human studies have been done to determine the adverse effects of resveratrol, all of them preliminary with small participant numbers. Adverse effects resulted mainly from long-term use (weeks or longer) and daily doses of 1000 mg or higher, causing nausea, stomach pain, flatulence, and diarrhea.[6] A review of 136 patients in seven studies who were given more than 500 mg for a month showed 25 cases of diarrhea, 8 cases of abdominal pain, 7 cases of nausea, and 5 cases of flatulence.[51] A 2018 review of resveratrol effects on blood pressure found that some people had increased frequency of bowel movements and loose stools.[16]
Occurrences
Plants
Resveratrol is a phytoalexin, a class of compounds produced by many plants when they are infected by pathogens or physically harmed by cutting, crushing, or ultraviolet radiation.[52]
Plants that synthesize resveratrol include knotweeds, pine trees including Scots pine and Eastern white pine, grape vines, raspberries, mulberries, peanut plants, cocoa bushes, and Vaccinium shrubs that produce berries, including blueberries, cranberries, and bilberries.[6][8][52]
Foods
The levels of resveratrol found in food varies considerably, even in the same food from season to season and batch to batch.[6]
Wine and grape juice
| Beverage | Resveratrol (μg/100 mL)[9] | |
|---|---|---|
| mean | range | |
| Red wine | 270 | 0 — 2780 |
| Rosé wine | 120 | 5 — 290 |
| White wine | 40 | 0 — 170 |
| Sparkling wine | 9 | 8 — 10 |
| Green grape juice | 5.08 | 0 — 10 |
Resveratrol concentrations in red wines average 1.9±1.7 mg trans-resveratrol/L (8.2±7.5 μM), ranging from nondetectable levels to 14.3 mg/L (62.7 μM) trans-resveratrol. Levels of cis-resveratrol follow the same trend as trans-resveratrol.[53]
In general, wines made from grapes of the Pinot noir and St. Laurent varieties showed the highest level of trans-resveratrol, though no wine or region can yet be said to produce wines with significantly higher concentrations than any other wine or region.[53] Champagne and vinegar also contain appreciable levels of resveratrol.[9]
Red wine contains between 0.2 and 5.8 mg/L, depending on the grape variety. White wine has much less because red wine is fermented with the skins, allowing the wine to extract the resveratrol, whereas white wine is fermented after the skin has been removed.[6] The composition of wine is different from that of grapes since the extraction of resveratrol from grapes depends on the duration of the skin contact, and the resveratrol 3-glucosides are in part hydrolysed, yielding both trans- and cis-resveratrol.[6][54]
Selected foods
| Food | Serving | Total resveratrol (mg)[6] |
|---|---|---|
| Peanuts (raw) | 1 cup (146 grams) | 0.01 – 0.26 |
| Peanut butter | 1 cup (258 grams) | 0.04 – 0.13 |
| Red grapes | 1 cup (160 grams) | 0.24 – 1.25 |
| Cocoa powder | 1 cup (200 grams) | 0.28 – 0.46 |
Ounce for ounce, peanuts have about 25% as much resveratrol as red wine.[6] Peanuts, especially sprouted peanuts, have a content similar to grapes in a range of 2.3 to 4.5 μg/g before sprouting, and after sprouting, in a range of 11.7 to 25.7 μg/g, depending on peanut cultivar.[9][52]
Mulberries (especially the skin) are a source of as much as 50 micrograms of resveratrol per gram dry weight.[55]
Most US supplements of resveratrol are derived from the root of Reynoutria japonica (also called Japanese knotweed, Hu Zhang, etc.)[6]
History
The first mention of resveratrol was in a Japanese article in 1939 by Michio Takaoka, who isolated it from Veratrum album, variety grandiflorum, and later, in 1963, from the roots of Japanese knotweed.[52][56][57][58] In 2004, Harvard University professor David Sinclair co-founded Sirtris Pharmaceuticals, the initial product of which was a resveratrol formulation.[59][60][61] Sirtris was purchased and made a subsidiary of GlaxoSmithKline in 2008 for $720 million and shut down in 2013, without successful drug development.[62][63]
Related compounds
- Dihydro-resveratrol
- Epsilon-viniferin, Pallidol and Quadrangularin A three different resveratrol dimers
- Elafibranor, a structurally related compound that acts as a dual PPARα/δ agonist
- THSG, a glycoside compound found in He Shou Wu which is very similar to resveratrol.
- Trans-diptoindonesin B, a resveratrol trimer
- Hopeaphenol, a resveratrol tetramer
- Oxyresveratrol, the aglycone of mulberroside A, a compound found in Morus alba, the white mulberry[64]
- Piceatannol, an active metabolite of resveratrol found in red wine
- Piceid, a resveratrol glucoside
- Pterostilbene, a doubly methylated resveratrol
- 4'-Methoxy-(E)-resveratrol 3-O-rutinoside, a compound found in the stem bark of Boswellia dalzielii[65]
- Rhaponticin a glucoside of the stilbenoid rhapontigenin, found in rhubarb rhizomes
See also
- Phenolic compounds in wine
- Polyphenol antioxidant
- Wine and health
- List of phytochemicals in food
- Nutrition
- Phytochemistry
- Secondary metabolites
References
- ↑ 1.0 1.1 Camont, Laurent; Cottart, Charles-Henry; Rhayem, Yara et al. (February 2009). "Simple spectrophotometric assessment of the trans-/cis-resveratrol ratio in aqueous solutions". Anal. Chim. Acta 634 (1): 121–128. doi:10.1016/j.aca.2008.12.003. PMID 19154820. Bibcode: 2009AcAC..634..121C.
- ↑ 2.0 2.1 "Resveratrol MSDS on Fisher Scientific website". http://www.fishersci.com/ecomm/servlet/msdsproxy?productName=AC430075000&productDescription=RESVERATROL+99%25+500MG&catNo=AC43007-5000&vendorId=VN00032119&s.
- ↑ Resveratrol MSDS on www.sigmaaldrich.com
- ↑ "Resveratrol amplifies profibrogenic effects of free fatty acids on human hepatic stellate cells". Hepatology Research 39 (6): 601–608. June 2009. doi:10.1111/j.1872-034X.2008.00485.x. PMID 19207580.
- ↑ GHS: Sigma-Aldrich R5010
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 6.12 "Resveratrol". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, OR. 11 June 2015. http://lpi.oregonstate.edu/infocenter/phytochemicals/resveratrol/.
- ↑ Fremont, Lucie (January 2000). "Biological Effects of Resveratrol". Life Sciences 66 (8): 663–673. doi:10.1016/S0024-3205(99)00410-5. PMID 10680575.
- ↑ 8.0 8.1 "Resveratrol in prostate diseases – a short review". Central European Journal of Urology 66 (2): 144–149. August 2013. doi:10.5173/ceju.2013.02.art8. PMID 24579014.
- ↑ 9.0 9.1 9.2 9.3 "Stilbenes-resveratrol in foods and beverages, version 3.6". Phenol-Explorer. 2016. http://phenol-explorer.eu/contents/polyphenol/592.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 "Resveratrol". MedlinePlus. 1 April 2019. https://medlineplus.gov/druginfo/natural/307.html.
- ↑ 11.0 11.1 "What is new for an old molecule? Systematic review and recommendations on the use of resveratrol". PLOS ONE 6 (6): e19881. 2011. doi:10.1371/journal.pone.0019881. PMID 21698226. Bibcode: 2011PLoSO...619881V.
- ↑ "Lack of efficacy of resveratrol on C-reactive protein and selected cardiovascular risk factors – Results from a systematic review and meta-analysis of randomized controlled trials". International Journal of Cardiology 189: 47–55. 2015. doi:10.1016/j.ijcard.2015.04.008. PMID 25885871.
- ↑ "Health benefits of resveratrol: Evidence from clinical studies". Med Res Rev 39 (5): 1851–1891. September 2019. doi:10.1002/med.21565. PMID 30741437.
- ↑ Zeraattalab-Motlagh, Sheida; Jayedi, Ahmad; Shab-Bidar, Sakineh (8 November 2021). "The effects of resveratrol supplementation in patients with type 2 diabetes, metabolic syndrome, and nonalcoholic fatty liver disease: an umbrella review of meta-analyses of randomized controlled trials". The American Journal of Clinical Nutrition 114 (5): 1675–1685. doi:10.1093/ajcn/nqab250. PMID 34320173.
- ↑ "Resveratrol in primary and secondary prevention of cardiovascular disease: a dietary and clinical perspective". Annals of the New York Academy of Sciences 1290 (1): 37–51. Jul 2013. doi:10.1111/nyas.12150. PMID 23855464. Bibcode: 2013NYASA1290...37T.
- ↑ 16.0 16.1 "Effect of resveratrol on blood pressure: A systematic review and meta-analysis of randomized, controlled, clinical trials". Critical Reviews in Food Science and Nutrition 58 (2): 1605–1618. January 2018. doi:10.1080/10408398.2017.1422480. PMID 29359958.
- ↑ "Effect of resveratrol on blood pressure: A meta-analysis of randomized controlled trials". Clinical Nutrition 34 (1): 27–34. March 2014. doi:10.1016/j.clnu.2014.03.009. PMID 24731650.
- ↑ "Resveratrol and cancer: focus on in vivo evidence". Endocr. Relat. Cancer 21 (3): R209–R225. June 2014. doi:10.1530/ERC-13-0171. PMID 24500760.
- ↑ "Resveratrol in metabolic health: an overview of the current evidence and perspectives". Annals of the New York Academy of Sciences 1290 (1): 74–82. Jul 2013. doi:10.1111/nyas.12141. PMID 23855468. Bibcode: 2013NYASA1290...74P.
- ↑ "Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus-systematic review and meta-analysis". Molecular Nutrition & Food Research 59 (1): 147–159. 19 August 2014. doi:10.1002/mnfr.201400173. PMID 25138371.
- ↑ De Ligt, M; Timmers, S; Schrauwen, P (2015). "Resveratrol and obesity: Can resveratrol relieve metabolic disturbances?". Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1852 (6): 1137–1144. doi:10.1016/j.bbadis.2014.11.012. PMID 25446988.
- ↑ "Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus – systematic review and meta-analysis". Mol Nutr Food Res 59 (1): 147–159. 2015. doi:10.1002/mnfr.201400173. PMID 25138371.
- ↑ Zhu, Xiangyun; Wu, Chunhua; Qiu, Shanhu; Yuan, Xuelu; Li, Ling (22 September 2017). "Effects of resveratrol on glucose control and insulin sensitivity in subjects with type 2 diabetes: systematic review and meta-analysis" (in en). Nutrition & Metabolism 14 (1): 60. doi:10.1186/s12986-017-0217-z. ISSN 1743-7075. PMID 29018489.
- ↑ Mousavi, S. M.; Milajerdi, A.; Sheikhi, A.; Kord-Varkaneh, H.; Feinle-Bisset, C.; Larijani, B.; Esmaillzadeh, A. (2019). "Resveratrol supplementation significantly influences obesity measures: a systematic review and dose–response meta-analysis of randomized controlled trials". Obesity Reviews 20 (3): 487–498. doi:10.1111/obr.12775. PMID 30515938.
- ↑ Asgary, Sedigheh; Karimi, Raheleh; Momtaz, Saeideh; Naseri, Rozita; Farzaei, Mohammad Hosein (1 June 2019). "Effect of resveratrol on metabolic syndrome components: A systematic review and meta-analysis". Reviews in Endocrine and Metabolic Disorders 20 (2): 173–186. doi:10.1007/s11154-019-09494-z. PMID 31065943.
- ↑ Koushki, Mehdi; Dashatan, Nasrin Amiri; Meshkani, Reza (July 2018). "Effect of Resveratrol Supplementation on Inflammatory Markers: A Systematic Review and Meta-analysis of Randomized Controlled Trials". Clinical Therapeutics 40 (7): 1180–1192.e5. doi:10.1016/j.clinthera.2018.05.015. PMID 30017172.
- ↑ Marx, Wolfgang; Kelly, Jaimon T.; Marshall, Skye; Cutajar, Jennifer; Annois, Brigitte; Pipingas, Andrew; Tierney, Audrey; Itsiopoulos, Catherine (1 June 2018). "Effect of resveratrol supplementation on cognitive performance and mood in adults: a systematic literature review and meta-analysis of randomized controlled trials". Nutrition Reviews 76 (6): 432–443. doi:10.1093/nutrit/nuy010. PMID 29596658. https://academic.oup.com/nutritionreviews/article/76/6/432/4954227.
- ↑ "Resveratrol for adults with type 2 diabetes mellitus". Cochrane Database Syst Rev 1 (1): CD011919. January 2020. doi:10.1002/14651858.CD011919.pub2. PMID 31978258.
- ↑ Asis, Marzieh; Hemmati, Niloufar; Moradi, Sajjad et al. (December 2019). "Effects of resveratrol supplementation on bone biomarkers: a systematic review and meta-analysis". Annals of the New York Academy of Sciences 1457 (1): 92–103. doi:10.1111/nyas.14226. PMID 31490554. Bibcode: 2019NYASA1457...92A.
- ↑ Baell, J; Walters, MA (25 September 2014). "Chemistry: Chemical con artists foil drug discovery". Nature 513 (7519): 481–483. doi:10.1038/513481a. PMID 25254460. Bibcode: 2014Natur.513..481B.
- ↑ Ingólfsson, HI; Thakur, P; Herold, KF et al. (15 August 2014). "Phytochemicals perturb membranes and promiscuously alter protein function". ACS Chemical Biology 9 (8): 1788–1798. doi:10.1021/cb500086e. PMID 24901212.
- ↑ Vang, O (August 2015). "Resveratrol: challenges in analyzing its biological effects". Annals of the New York Academy of Sciences 1348 (1): 161–170. doi:10.1111/nyas.12879. PMID 26315294. Bibcode: 2015NYASA1348..161V.
- ↑ "High absorption but very low bioavailability of oral resveratrol in humans". Drug Metab. Dispos. 32 (12): 1377–1382. December 2004. doi:10.1124/dmd.104.000885. PMID 15333514.
- ↑ "Bioactivity of dietary polyphenols: The role of metabolites". Critical Reviews in Food Science and Nutrition 60 (4): 626–659. 2020. doi:10.1080/10408398.2018.1546669. PMID 30614249.
- ↑ 35.0 35.1 "Therapeutic potential of resveratrol: the in vivo evidence". Nature Reviews Drug Discovery 5 (6): 493–506. 2006. doi:10.1038/nrd2060. PMID 16732220.
- ↑ Sharan S, Nagar S; Nagar (2013). "Pulmonary metabolism of resveratrol: In vitro and in vivo evidence". Drug Metabolism and Disposition 41 (5): 1163–1169. doi:10.1124/dmd.113.051326. PMID 23474649.
- ↑ "Progress to Improve Oral Bioavailability and Beneficial Effects of Resveratrol". International Journal of Molecular Sciences 20 (6): 1381. 2019. doi:10.3390/ijms20061381. PMID 30893846.
- ↑ "Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans". Oxidative Medicine and Cellular Longevity 2015: 837042. 2015. doi:10.1155/2015/837042. PMID 26221416.
- ↑ 39.0 39.1 "Isolation, characterization, and evolution in red wine vinification of resveratrol monomers". Journal of Agricultural and Food Chemistry 43 (7): 1820–1823. 1995. doi:10.1021/jf00055a013.
- ↑ "Direct HPLC Analysis of cis- and trans-Resveratrol and Piceid Isomers in Spanish Red Vitis vinifera Wines". Journal of Agricultural and Food Chemistry 43 (2): 281–283. 1995. doi:10.1021/jf00050a003.
- ↑ Resveratrol Photoisomerization: An Integrative Guided-Inquiry Experiment Elyse Bernard, Philip Britz-McKibbin, Nicholas Gernigon Vol. 84 No. 7 July 2007 Journal of Chemical Education 1159.
- ↑ "Photochemical generation of a new, highly fluorescent compound from non-fluorescent resveratrol". Chemical Communications 48 (32): 3839–3841. 2012. doi:10.1039/C2CC30940H. PMID 22436889.
- ↑ "Resveratrol and its glycon piceid are stable polyphenols". J Med Food 9 (1): 11–14. 2006. doi:10.1089/jmf.2006.9.11. PMID 16579722.
- ↑ "Stability of trans-resveratrol associated with transport proteins". J Agric Food Chem 62 (19): 4384–4391. 2014. doi:10.1021/jf405584a. PMID 24773207.
- ↑ "Stability of resveratrol over time and in the various stages of grape transformation". Drugs Exp Clin Res 24 (4): 207–211. 1998. PMID 10051967.
- ↑ 46.0 46.1 Valletta, Alessio; Iozia, Lorenzo Maria; Leonelli, Francesca (January 2021). "Impact of Environmental Factors on Stilbene Biosynthesis" (in en). Plants 10 (1): 90. doi:10.3390/plants10010090. PMID 33406721.
- ↑ 47.0 47.1 Dubrovina, A. S.; Kiselev, K. V. (October 2017). "Regulation of stilbene biosynthesis in plants" (in en). Planta 246 (4): 597–623. doi:10.1007/s00425-017-2730-8. ISSN 0032-0935. PMID 28685295. Bibcode: 2017Plant.246..597D. http://link.springer.com/10.1007/s00425-017-2730-8.
- ↑ Wang, Chuanhong; Zhi, Shuang; Liu, Changying et al. (2017). "Characterization of Stilbene Synthase Genes in Mulberry (Morus atropurpurea) and Metabolic Engineering for the Production of Resveratrol in Escherichia coli". Journal of Agricultural and Food Chemistry 65 (8): 1659–1668. doi:10.1021/acs.jafc.6b05212. PMID 28168876.
- ↑ "Dimerization of resveratrol by the grapevine pathogen Botrytis cinerea". J. Nat. Prod. 63 (1): 29–33. January 2000. doi:10.1021/np990266n. PMID 10650073.
- ↑ Cichewicz RH, Kouzi SA; Kouzi (October 1998). "Biotransformation of resveratrol to piceid by Bacillus cereus". J. Nat. Prod. 61 (10): 1313–1314. doi:10.1021/np980139b. PMID 9784180.
- ↑ "Review of recent data on the metabolism, biological effects, and toxicity of resveratrol in humans". Molecular Nutrition & Food Research 58 (1): 7–21. 2014. doi:10.1002/mnfr.201200589. PMID 23740855.
- ↑ 52.0 52.1 52.2 52.3 Sales, JM; Resurreccion, AV (2014). "Resveratrol in peanuts". Critical Reviews in Food Science and Nutrition 54 (6): 734–770. doi:10.1080/10408398.2011.606928. PMID 24345046.
- ↑ 53.0 53.1 "A review of the content of the putative chemopreventive phytoalexin resveratrol in red wine". Food Chemistry 101 (2): 449–457. 2007. doi:10.1016/j.foodchem.2006.01.047.
- ↑ Naiker, M.; Anderson, S.; Johnson, J. B.; Mani, J. S.; Wakeling, L.; Bowry, V. (2020-07-21). "Loss of trans -resveratrol during storage and ageing of red wines" (in en). Australian Journal of Grape and Wine Research 26 (4): 385–387. doi:10.1111/ajgw.12449. ISSN 1322-7130.
- ↑ "Resveratrol: a candidate nutritional substance for prostate cancer prevention". J. Nutr. 133 (7 Suppl): 2440S–2443S. July 2003. doi:10.1093/jn/133.7.2440S. PMID 12840221.
- ↑ Takaoka M (1939). "Resveratrol, a new phenolic compound, from Veratrum grandiflorum". Journal of the Chemical Society of Japan 60 (11): 1090–1100. doi:10.1246/nikkashi1921.60.1090. http://www.mendeley.com/research/resveratrol-new-phenolic-compound-veratrum-grandiflorum/.
- ↑ Takaoka, Michio (1940). "The Phenolic Substances of White Hellebore (Veratrum Grandiflorum Loes. Fill). V". Nippon Kagaku Kaishi 61 (10): 1067–1069. doi:10.1246/nikkashi1921.61.1067.
- ↑ Nonomura; Kanagawa (1963). "Chemical constituents of Polygonaceous plants. I. studies on the components of Ko-jo-kon. (Polygonum cuspidatum SIEB et ZUCC)". Yakugaku Zasshi 83 (10): 988–990. doi:10.1248/yakushi1947.83.10_988.
- ↑ Rimas A (2006-12-11). "His research targets the aging process". The Boston Globe. http://www.boston.com/news/globe/health_science/articles/2006/12/11/his_research_targets_the_aging_process/.
- ↑ Stipp D (2007-01-19). "Can red wine help you live forever?". Fortune magazine. https://money.cnn.com/2007/01/18/magazines/fortune/Live_forever.fortune/index.htm?postversion=2007011912.
- ↑ Weintraub A (2009-07-29). "Resveratrol: The Hard Sell on Anti-Aging". Bloomberg Businessweek. http://www.businessweek.com/magazine/content/09_32/b4142000175800.htm.
- ↑ Carroll, John; McBride, Ryan (Mar 12, 2013). "Updated: GSK moves to shutter Sirtris' Cambridge office, integrate R&D" (in en). FierceBiotech. http://www.fiercebiotech.com/r-d/updated-gsk-moves-to-shutter-sirtris-cambridge-office-integrate-r-d.
- ↑ "GSK absorbs controversial 'longevity' company: News blog". Nature Blog. http://blogs.nature.com/news/2013/03/gsk-absorbs-controversial-longevity-company.html..
- ↑ "Biotransformation of mulberroside A from Morus alba results in enhancement of tyrosinase inhibition". J. Ind. Microbiol. Biotechnol. 37 (6): 631–637. June 2010. doi:10.1007/s10295-010-0722-9. PMID 20411402.
- ↑ Alemika Taiwo E, Onawunmi Grace O and Olugbade Tiwalade O, Antibacterial phenolics from Boswellia dalzielii. Nigerian Journal of Natural Products and Medicines, 2006
External links
 |
