Chemistry:Kappa-carbide
κ-Carbides are a special class of carbide structures. They are most known for appearing in steels containing manganese and aluminium where they have the molecular formula (Fe,Mn)3AlC.[1]
Properties
Structure
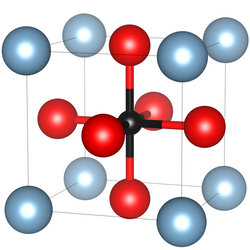
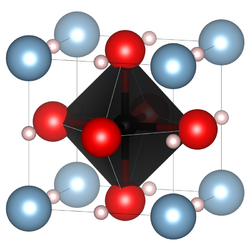
κ-Carbides crystallise in the perovskite structure type with the space group Pm3m (Nr. 221).[2] This structure was, inter alia, elucidated with XRD-measurements on steel alloys containing κ-carbide precipitates but also on single crystals of manganese-κ-carbides with a molecular formula of Mn3.1Al0.9C and a lattice parameter of a=3.87Å.[3] In steel alloys where diverse arrangements of the atoms are possible, a considerable effect of the short range ordering, e.g. of iron and manganese on the microscopic properties of the alloy, has been observed.[4] This is especially important for the role as hydrogen-traps in steels.[5]
Composition
A first glance at the composition of a steel alloy is achieved by analysing its surface with EDX-technique.[3]
Depending on the content of the alloying elements of the steel, different types of κ-carbides can form. They occur in both ferritic (α-Fe) and austenitic (γ-Fe) steels.[1] Typical alloying elements are iron, manganese, aluminium, carbon, and silicon.[2][6]
Magnetism
SQUID measurements on polycrystalline Mn3.1Al0.9C revealed a soft ferromagnetic behaviour of this κ-carbide with a Curie temperature of 295±13 K, a remanent magnetic moment of 3.22 μB and a coercive field of 1.9 mT.[3] DFT-simulations confirmed these findings and indicated that other κ-carbides behave similarly.[7]
Occurrence
κ-carbides are typically found as precipitates in high-performance steels.[8] A common example is the TRIPLEX steel with the generic composition FexMnyAlzC containing 18-28 % manganese, 9-12 % aluminium and 0.7-1.2 % carbon (in mass %).[9] It is a high-strength, low-density steel consisting of austenitic γ–Fe(Mn,Al,C) solid solution, nano size κ-carbides (Fe,Mn)3AlC1-x and α–Fe(Al,Mn) ferrite.[9] Other similar steels are known for their high ductility.[4] κ-carbides are usually formed from areas enriched in carbon through spinodal decomposition and are key determinants of the properties of these steels.[10] The low density is e.g. obtained after a hot rolling post-process.[1] Upon cooling, different domains of austenite and ferrite are formed and κ-carbides form at the boundaries of these domains.[11] Continuing the cooling process leads to a phase transition of austenite to ferrite and the κ-carbides are released as a result of an eutectoid transformation in form of a precipitate.[11]
The κ-carbides can have an additional strengthening effect on steels[5] because they can function as a hydrogen trap to counteract hydrogen embrittlement.[3] Ab-initio DFT-simulations have shown that hydrogen can occupy the same site as carbon in the κ-carbide precipitates or an initially empty interstitial lattice site. Hereby, it was found that an increased Mn content enhances the H-trapping by attractive short-range interactions. The aforementioned short-range ordering of Fe and Mn in the κ-carbide has a significant influence on the strength of this effect.[5] This behaviour can be used as an additional method to cope with hydrogen embrittlement which is normally prevented by simply minimising the contact of metal and hydrogen.[4]
See also
- Contemporary steel
- Carbon steel
- Alloy steels
References
- ↑ 1.0 1.1 1.2 Sozańska-Jędrasik, Liwia; Mazurkiewicz, Janusz; Matus, Krzysztof; Borek, Wojciech (6 February 2020). "Structure of Fe-Mn-Al-C Steels after Gleeble Simulations and Hot-Rolling". Materials 13 (3): 739. doi:10.3390/ma13030739. PMID 32041206. Bibcode: 2020Mate...13..739S.
- ↑ 2.0 2.1 Seol, Jae Bok (28 December 2018). "A Brief Review of κ-Carbide in Fe-Mn-Al-C Model Alloys". Applied Microscopy 48 (4): 117–121. doi:10.9729/am.2018.48.4.117.
- ↑ 3.0 3.1 3.2 3.3 Dierkes, Hannes; van Leusen, Jan; Bogdanovski, Dimitri; Dronskowski, Richard (17 January 2017). "Synthesis, Crystal Structure, Magnetic Properties, and Stability of the Manganese-Rich "Mn3AlC" κ Phase". Inorganic Chemistry 56 (3): 1045–1048. doi:10.1021/acs.inorgchem.6b02816. PMID 28094520.
- ↑ 4.0 4.1 4.2 Song, Wenwen; Bogdanovski, Dimitri; Yildiz, Ahmet; Houston, Judith; Dronskowski, Richard; Bleck, Wolfgang (10 January 2018). "On the Mn–C Short-Range Ordering in a High-Strength High-Ductility Steel: Small Angle Neutron Scattering and Ab Initio Investigation". Metals 8 (1): 44. doi:10.3390/met8010044.
- ↑ 5.0 5.1 5.2 Timmerscheidt, Tobias; Dey, Poulumi; Bogdanovski, Dimitri; von Appen, Jörg; Hickel, Tilmann; Neugebauer, Jörg; Dronskowski, Richard (11 July 2017). "The Role of κ-Carbides as Hydrogen Traps in High-Mn Steels". Metals 7 (7): 264. doi:10.3390/met7070264.
- ↑ Bartlett, Laura N.; Van Aken, David C.; Medvedeva, Julia; Isheim, Dieter; Medvedeva, Nadezhda I.; Song, Kai (20 February 2014). "An Atom Probe Study of Kappa Carbide Precipitation and the Effect of Silicon Addition". Metallurgical and Materials Transactions A 45 (5): 2421–2435. doi:10.1007/s11661-014-2187-3. Bibcode: 2014MMTA...45.2421B.
- ↑ Seo, Seung-Wo. "First Principles Calculations on Thermodynamic Properties and Magnetism of k-carbide and Monte-Carlo Cell Gas Model". https://www.phase-trans.msm.cam.ac.uk/2011/SEO_Thesis.pdf. Retrieved 14 July 2020.
- ↑ Gutierrez-Urrutia, I.; Raabe, D. (March 2013). "Influence of Al content and precipitation state on the mechanical behavior of austenitic high-Mn low-density steels". Scripta Materialia 68 (6): 343–347. doi:10.1016/j.scriptamat.2012.08.038.
- ↑ 9.0 9.1 Frommeyer, Georg; Brüx, Udo (September 2006). "Microstructures and Mechanical Properties of High-Strength Fe-Mn-Al-C Light-Weight TRIPLEX Steels". Steel Research International 77 (9–10): 627–633. doi:10.1002/srin.200606440.
- ↑ Rana, Radhakanta; Lahaye, Chris; Ray, Ranjit Kumar (29 August 2014). "Overview of Lightweight Ferrous Materials: Strategies and Promises". JOM 66 (9): 1734–1746. doi:10.1007/s11837-014-1126-5. Bibcode: 2014JOM....66i1734R.
- ↑ 11.0 11.1 Akdeniz, M. Vedat (26 August 2008). "Solidification Microstructures and Carbides Morphology in Rapidly Solidified Fe-Al-Cr-C Alloys". Metals and Materials International 14 (4): 397–402. doi:10.3365/met.mat.2008.08.397. Bibcode: 2008MMI....14..397A.
External links
- [1] κ Carbide in Steels] (Phase Transformations & Complex
Properties Research Group, University of Cambridge)
 |