Chemistry:Laccaic acid
| |
| Names | |
|---|---|
| IUPAC name
7-[5-(2-acetamidoethyl)-2-hydroxyphenyl]-3,5,6,8-tetrahydroxy-9,10-dioxoanthracene-1,2-dicarboxylic acid
| |
| Other names
Red lac, Shellac, Lac dye
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C₂₆H₁₉NO₁₂ | |
| Molar mass | 537.44 g·mol−1 |
| Density | 1.7±0.1 g.cm3 |
| Boiling point | 995.3±65.0 °C at 760 mmHg |
| Vapor pressure | 0.0±0.3 mmHg at 25°C |
| Hazards | |
| Flash point | 555.7±34.3 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
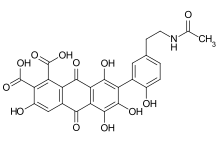
Laccaic acids or laccainic acids are a group of five anthraquinone derivatives, rated from A to E, constituting the red shellac obtained from the cochineal Kerria lacca, just like carminic acid or kermesic acid.[1] For this article, it will mostly concentrate on the laccaic acid A (LCA).[2][3]
History
Etymology
The word varnish goes back to the old Indian Sanskrit word laksha, meaning a hundred thousand lice, and came into German via the Italian “lacca” in the 16th century. The word also exists in Hindi (Lakh) and Sinhala (Lakda). The term Lac Dye comes from English "dye" means paint dye. This pigment is mostly found in South and South-East Asia, getting its name and area of use from there.
Usage
It is mainly used to dye natural fabrics (mostly silk, wool, or cotton) and food, whether it be drinks or solid products.[4] It is one of the most used natural dye but it is less used in cosmetics as carmine is still the main natural dye used in this industry. The bright red colorant gives a lightfast tint to silk and wool. It is a similar color to dyes obtained from cochineals and kermes. The color of the dye can be modified by the choice of mordant from violet to red to brown. The use of lac dye can be traced back to 250 AD when it was mentioned by Claudius Aelianus, a Roman writer on a volume about natural history. This pigment made from lac dye, Indian Lake, was listed by Winsor & Newton in their 1896 catalogue.
Derivatives
These derivatives differ through one ramification except for the acid D, which is closer in form to the kermesic acid. Acid laccaic D can be confused or interchanged with flavokermisic acid due to their almost identical structure.[5] These acids can all be represented in a general form (lac-dye), where the derivative A is the most important.
The differents derivatives:
Acid B (3,5,6,8-tetrahydroxy-7-[2-hydroxy-5-(2-hydroxyethyl)phenyl]-9,10-dioxoanthracene-1,2-dicarboxylic acid)[6]
Acid C (7-[5-(2-amino-2-carboxyethyl)-2-hydroxyphenyl]-3,5,6,8-tetrahydroxy-9,10-dioxoanthracene-1,2-dicarboxylic acid)[7]
Acid D (3,6,8-trihydroxy-1-methyl-9,10-dioxoanthracene-2-carboxylic acid)[8]
Acid E (7-[5-(2-aminoethyl)-2-hydroxyphenyl]-3,5,6,8-tetrahydroxy-9,10-dioxoanthracene-1,2-dicarboxylic acid)[9]
Structure
| Laccaic acids | |||||
| Name | Laccaic acid A | Laccaic acid B | Laccaic acid C | Laccaic acid D | Laccaic acid E |
| Structure | 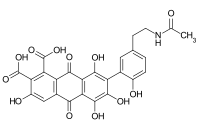 |
200px | 200px | 200px | 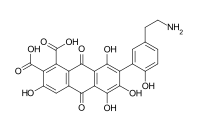
|
| Molecular formula | C26H19NO12 | C24H16O12 | C25H17NO13 | C16H10O7 | C24H17NO11 |
| Molecular weight | 537,44 g·mol−1 | 496,38 g·mol−1 | 539,41 g·mol−1 | 314,25 g·mol−1 | 495,40 g·mol−1 |
| CAS number | 15979-35-8 | 17249-00-2 | 23241-56-7 | 18499-84-8 | 14597-16-1 |
| 60687-93-6 (A-E mix) | |||||
Laccaic acid A has an amide function in its ramification while the acids B, C, E have amine functions[10]
The only difference between the acid D and kermesic acid is a hydroxide function missing on the position 8
Isolation/Extraction
Acids A, B, C and E can be isolated from lac dye through many different ways : microwave induced from lac insects, high speed counter-current chromatography or liquid chromatography-mass spectrometry.[11][12][13]
Extraction from the stick lac
The stick lac collected from Rain trees (mostly located in Asia) can be powdered in a mill and finely ground. The powdered material is extracted with deionized water at 60°C for 1 h. The aqueous solution is filtered and then concentrated under reduced pressure in a rotary evaporator to give a crude lac dye extract, which can then be used without further purification. This extract can be directly named lac dye.[14]
Extraction from insect corpse
Grind the body with the appropriate amount of water, then use 4-5 times the original amount of water for extraction several times. Centrifuge to remove all slag, then add a small amount of sodium hydroxide and calcium chloride solution to the extract, then add dilute chloric acid. The pH slowly adjusts to 2.1. Let stand for 3-4 hours, clean and filter. Concentrated sulfuric acid is added to the filtrate until the pigment crystals precipitate, filtered through spun silk (~0.12 mm), the filtrate is allowed to stand for 1-2 days, and then the pigment crystals are precipitated, filtered and washed 3 times by water , dried crush and sift at 60 °C, resulting in a final product with a degree of separation of ~0.8%.
Color water extraction method
After the dye washing of the wastewater from the shellac cleaning, the pH value of the solution is adjusted to 4.0-4.5 with dilute hydrochloric acid. The supernatant is taken for filtration after standing for 4 hours, the pH of the filtrate is adjusted to 5.5-6.0 with a dilute alkaline solution, then a saturated calcium chloride solution is added to precipitate calcium-lac acid. After 8 hours of clarification . The supernatant is removed, the precipitate is filtered, after filtration, concentrated hydrochloric acid is added, washed with water until the acid is no longer present, and dried to obtain the final lac red pigment.[15]
Bio-Chemical Properties
DNMT1 is inhibited by LCA which has a stronger inhibitory effect than SG-1027 (HY-13962). LCA is a class of DNMT inhibitors may be a useful mechanism to inhibit DNMT.[16]
Utilisation
As a major component of lac dye, LCA is usually chosen as a representative for the lac dye to describe their thermodynamics properties including adsorption, dyeability, fastness and shade variation of lac dyeing on silk and cotton. Studies indicate that the intermolecular interactions between LCA and fibers as well as between LCA and mordants play a key role on the adsorption and dyeability of lac dye[17][18]
Dyeing fabric
Lac extract produces purple colors from burgundy to deep purple. The colors are similar to cochineal colors, but warmer, softer and more muted. Lac paint has high light and washing fastness on silk and wool. Only small amounts are needed for medium depth shade. Lac is not so fast in cellulose fibers (plant fibers). Lac is very sensitive to pH, increasing alkalinity will turn the colors plummy purple, while acidity will give bright oranges. However, colors that have been altered by the pH change may turn red again after washing.
References
- ↑ PubChem. "Kermesic Acid" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/11727234.
- ↑ PubChem. "Laccaic acid A" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/5491415.
- ↑ "laccaic acid A | C26H19NO12 | ChemSpider". http://www.chemspider.com/Chemical-Structure.4590519.html.
- ↑ Park, Jin-Sung; Kim, Seung-Hyun; Kim, Yun-Soon; Kwon, Euna; Lim, Hyun-Jin; Han, Kang-Min; Choi, Yang-Kyu; Jung, Chul-Woo et al. (2022-10-31). "Nonclinical safety evaluation of food colorant lac dye via systematic toxicity profiling with assessment of in vivo antigenic potential". Frontiers in Pharmacology 13: 1020379. doi:10.3389/fphar.2022.1020379. ISSN 1663-9812. PMID 36386152.
- ↑ Wouters, Jan; Verhecken, André (1987-01-01). "The chemical nature of flavokermesic acid" (in en). Tetrahedron Letters 28 (11): 1199–1202. doi:10.1016/S0040-4039(00)95325-5. ISSN 0040-4039. https://www.sciencedirect.com/science/article/pii/S0040403900953255.
- ↑ PubChem. "Laccaic acid B" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/5491366.
- ↑ PubChem. "Laccaic acid C" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/9828689.
- ↑ PubChem. "Laccaic acid D" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/9883304.
- ↑ PubChem. "Laccaic acid E" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/15045881.
- ↑ Cooksey, C. J. (2019-02-17). "The red insect dyes: carminic, kermesic and laccaic acids and their derivatives" (in en). Biotechnic & Histochemistry 94 (2): 100–107. doi:10.1080/10520295.2018.1511065. ISSN 1052-0295. PMID 30354531. https://www.tandfonline.com/doi/full/10.1080/10520295.2018.1511065.
- ↑ Oka, Hisao; Ito, Yuko; Yamada, Sadaji; Kagami, Tadaaki; Hayakawa, Junko; Harada, Ken-ichi; Atsumi, Eiichiro; Suzuki, Masanao et al. (1998-07-10). "Separation of lac dye components by high-speed counter-current chromatography" (in en). Journal of Chromatography A 813 (1): 71–77. doi:10.1016/S0021-9673(98)00311-2. ISSN 0021-9673. PMID 9697316. https://www.sciencedirect.com/science/article/pii/S0021967398003112.
- ↑ Ghosh, Manik; Dey, Suddhasattya (2019-10-14). "Bioactivity-guided isolation of Laccaic Acid-A: A potent anti-cancer agent from Laccifer lacca (Kerr)" (in en). Indian Journal of Traditional Knowledge 18 (4): 677–685. doi:10.56042/ijtk.v18i4.28998. ISSN 0975-1068. http://op.niscpr.res.in/index.php/IJTK/article/view/28998.
- ↑ Adeel, Shahid; Hussaan, Muhammad; Rehman, Fazal-ur; Habib, Noman; Salman, Mahwish; Naz, Saba; Amin, Nimra; Akhtar, Nasim (2018-03-20). "Microwave-assisted sustainable dyeing of wool fabric using cochineal-based carminic acid as natural colorant". Journal of Natural Fibers 16 (7): 1026–1034. doi:10.1080/15440478.2018.1448317. ISSN 1544-0478. http://dx.doi.org/10.1080/15440478.2018.1448317.
- ↑ Dingyu Hu; Akira Hasegawa; Shin-ichi Nakatsuka (1997-08-01). "Isolation and Structure Determination of Laccaic Acid F from Lac-Dye Produced from Thai Sticklac" (in en). Heterocyclic Communications 3 (4): 327–330. doi:10.1515/HC.1997.3.4.327. ISSN 2191-0197.
- ↑ "LACCAIC ACID". https://www.chembk.com/en/chem/LACCAIC%20ACID.
- ↑ Fagan, Rebecca L.; Cryderman, Diane E.; Kopelovich, Levy; Wallrath, Lori L.; Brenner, Charles (16 August 2013). "Laccaic Acid A Is a Direct, DNA-competitive Inhibitor of DNA Methyltransferase 1 *" (in English). Journal of Biological Chemistry 288 (33): 23858–23867. doi:10.1074/jbc.M113.480517. ISSN 0021-9258. PMID 23839987. PMC 3745332. https://www.jbc.org/article/S0021-9258(20)45276-7/fulltext. Retrieved 25 March 2023.
 This article incorporates text from this source, which is available under the CC BY 4.0 license.
This article incorporates text from this source, which is available under the CC BY 4.0 license.
- ↑ Kongkachuichay, Paisan; Shitangkoon, Aroonsiri; Chinwongamorn, Nontalee (2002-05-01). "Thermodynamics of adsorption of laccaic acid on silk" (in en). Dyes and Pigments 53 (2): 179–185. doi:10.1016/S0143-7208(02)00014-1. ISSN 0143-7208. https://www.sciencedirect.com/science/article/pii/S0143720802000141.
- ↑ "Material-Archiv". https://materialarchiv.ch/de/ma:material_1013.
 |




