Chemistry:Mavacamten
 | |
| Clinical data | |
|---|---|
| Trade names | Camzyos |
| Other names | MYK-461 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a622047 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Cardiac myosin inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
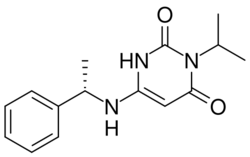
| Formula | C15H19N3O2 |
| Molar mass | 273.336 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Mavacamten, sold under the brand name Camzyos, is a medication used to treat obstructive hypertrophic cardiomyopathy.[5][6]
Mavacamten is a small-molecule allosteric [7] and cardiac myosin inhibitor.[5] It was developed by MyoKardia, a subsidiary of Bristol Myers Squibb.[8] In clinical studies, mavacamten has demonstrated significant efficacy in reducing cardiac muscle contractility by targeting the sarcomere hypercontractility that is one of the characteristics of hypertrophic cardiomyopathy and inhibits excessive myosin actin cross-bridge formation, shifting the overall myosin population towards an energy-sparing, recruitable, super-relaxed state.[5]
Mavacamten was approved for medical use in the United States in April 2022.[5][9][10][11] The US Food and Drug Administration (FDA) considers it to be a first-in-class medication.[12][13]
Medical uses
Mavacamten is indicated for the treatment of adults with symptomatic New York Heart Association class II-III obstructive hypertrophic cardiomyopathy to improve functional capacity and symptoms.[5]
Adverse effects
The US prescribing information for mavacamten contains a boxed warning regarding heart failure. Mavacamten reduces LVEF and can cause heart failure due to systolic dysfunction.[5]
History
Mavacamten was granted orphan drug designation by the US Food and Drug Administration (FDA).[14]
Society and culture
Legal status
On 26 April 2023, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Camzyos, intended for the treatment of symptomatic obstructive hypertrophic cardiomyopathy (oHCM).[15] The applicant for this medicinal product is Bristol-Myers Squibb Pharma EEIG.[15] In June 2023, the European Commission approved Mavacamten.[6][16][17]
Mavacamten is approved for use in the US,[18] Canada,[19] Australia,[20] South Korea,[21] Singapore,[22] Switzerland,[23] Brazil[24] and Macau.[25]
Names
Mavacamten is the international nonproprietary name (INN).[26]
References
- ↑ 1.0 1.1 "Camzyos". Therapeutic Goods Administration (TGA). 13 October 2022. https://www.tga.gov.au/resources/auspmd/camzyos.
- ↑ "Camzyos (Bristol-Myers Squibb Australia Pty Ltd)". Therapeutic Goods Administration (TGA). 7 October 2022. https://www.tga.gov.au/resources/prescription-medicines-registrations/camzyos-bristol-myers-squibb-australia-pty-ltd.
- ↑ "Camzyos Product information". https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=102157.
- ↑ "Summary Basis of Decision for Camzyos". 12 April 2023. https://dhpp.hpfb-dgpsa.ca/review-documents/resource/SBD1684173887386.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 "Camzyos- mavacamten capsule, gelatin coated". 28 April 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=669c936b-3ee6-4e36-8a22-79dd11b1255b.
- ↑ 6.0 6.1 6.2 "Camzyos EPAR". 24 July 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/camzyos.
- ↑ "The Impact of Mavacamten on the Pathophysiology of Hypertrophic Cardiomyopathy: A Narrative Review". American Journal of Cardiovascular Drugs 22 (5): 497–510. September 2022. doi:10.1007/s40256-022-00532-x. PMID 35435607.
- ↑ "Bristol Myers Squibb Completes Acquisition of MyoKardia, Strengthening Company's Leading Cardiovascular Franchise" (Press release). Bristol Myers Squibb. 17 November 2020. Archived from the original on 29 April 2022. Retrieved 29 April 2022 – via Business Wire.
- ↑ "FDA approves new drug to improve heart function in adults with rare heart condition" (Press release). U.S. Food and Drug Administration (FDA). 29 April 2022. Archived from the original on 29 November 2022. Retrieved 29 November 2022.
- ↑ "FDA approves Bristol Myers' oral heart disease drug". Reuters. 29 April 2022. https://www.reuters.com/business/healthcare-pharmaceuticals/fda-approves-bristol-myers-heart-disease-drug-2022-04-29/.
- ↑ "U.S. Food and Drug Administration Approves Camzyos (mavacamten) for the Treatment of Adults With Symptomatic New York Heart Association Class II-III Obstructive Hypertrophic Cardiomyopathy (HCM) to Improve Functional Capacity and Symptoms" (Press release). Bristol Myers Squibb. 28 April 2022. Archived from the original on 29 April 2022. Retrieved 29 April 2022 – via Business Wire.
- ↑ "Advancing Health Through Innovation: New Drug Therapy Approvals 2022". 10 January 2023. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/new-drug-therapy-approvals-2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ (PDF) New Drug Therapy Approvals 2022 (Report). January 2024. https://www.fda.gov/media/164429/download. Retrieved 14 January 2024.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Mavacamten Orphan Drug Designations and Approvals". 27 April 2016. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=515015.
- ↑ 15.0 15.1 "Camzyos: Pending EC decision". 26 April 2023. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/camzyos. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Camzyos". 27 June 2023. https://ec.europa.eu/health/documents/community-register/html/h1716.htm.
- ↑ Priyan, Vishnu (27 June 2023). "EC approves BMS' Camzyos for hypertrophic cardiomyopathy". https://www.pharmaceutical-technology.com/news/ec-approves-bms-camzyos/.
- ↑ "FDA approves new drug to improve heart function in adults with rare heart condition". U.S. Food and Drug Administration (FDA). 29 April 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-new-drug-improve-heart-function-adults-rare-heart-condition.
- ↑ "Health Canada Approves Camzyos (mavacamten capsules) for the Treatment of Adults with Symptomatic Obstructive Hypertrophic Cardiomyopathy". Bristol Myers Squibb (Press release). 10 November 2022. Retrieved 27 June 2023.
- ↑ "Camzyos". Therapeutic Goods Administration (TGA). 13 October 2022. https://www.tga.gov.au/resources/auspmd/camzyos.
- ↑ "의약품안전나라" (in ko). https://nedrug.mfds.go.kr/.
- ↑ "LianBio Announces Marketing Approval of Camzyos (mavacamten) in Singapore". https://www.biospace.com/article/lianbio-announces-marketing-approval-of-camzyos-mavacamten-in-singapore/.
- ↑ "Camzyos, Hartkapseln (Mavacamtenum)". https://www.swissmedic.ch/swissmedic/en/home/humanarzneimittel/authorisations/new-medicines/camzyos-hartkapseln-mavacamtenum.html.
- ↑ "Camzyos (mavacanteno): novo registro" (in pt-br). https://www.gov.br/anvisa/pt-br/assuntos/medicamentos/novos-medicamentos-e-indicacoes/camzyos-r-mavacanteno-novo-registro.
- ↑ "LianBio Announces Marketing Approval of Camzyos (mavacamten) in the Macau Special Administrative Region (SAR) of China" (Press release). LianBio. 11 May 2023. Retrieved 28 June 2023 – via GlobeNewswire.
- ↑ "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 78". WHO Drug Information 31 (3). 2017.
Further reading
- "Assessing health-related quality-of-life in patients with symptomatic obstructive hypertrophic cardiomyopathy: EQ-5D-based utilities in the EXPLORER-HCM trial". Journal of Medical Economics 25 (1): 51–58. 2022. doi:10.1080/13696998.2021.2011301. PMID 34907813.
External links
- Clinical trial number NCT03470545 for "Clinical Study to Evaluate Mavacamten (MYK-461) in Adults With Symptomatic Obstructive Hypertrophic Cardiomyopathy (EXPLORER-HCM)" at ClinicalTrials.gov
 |
