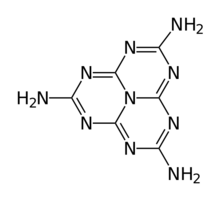
Chemistry:Melem
| |
| Names | |
|---|---|
| IUPAC name
11-imino-2,4,6,8,10,12,13-heptazatricyclo[7.3.1.05,13]trideca-1(12),2,4,7,9-pentaene-3,7-diamine
| |
| Other names
2,5,8-triamino-heptazine; 2,5,8-triamino-tri-s-triazine; cyamelurotriamide; triamino-s-heptazine; 1,3,4,6,7,9,9b-Heptaazaphenalene-2,5,8-triamine
| |
| Identifiers | |
3D model (JSmol)
|
|
| 27284 | |
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| 241276 | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H6N10 | |
| Molar mass | 218.18 g/mol |
| Appearance | white solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
In chemistry, melem is a compound with formula C6N10H6; specifically, 2,5,8-triamino-heptazine or 2,5,8-triamino-tri-s-triazine, whose molecule can be described as that of heptazine with the three hydrogen atoms replaced by amino groups. It is a white crystalline solid.[1]
Preparation
Melem can be prepared by thermal decomposition of various C−N−H compounds, such as melamine C3N3(NH2)3, dicyandiamide H4C2N4, ammonium dicyanamide NH4[N(CN)2], cyanamide H2CN2, at 400 to 450 °C.[1][2]
Structure and properties
Crystal structure
Melem crystallizes in the group P21/c (No. 14), with parameters a = 739.92(1) pm, b = 865.28(3) pm, c = 1338.16(4) pm, β = 99.912(2)°, and Z = 4. The almost-planar molecules are arranged in parallel layers spaced 327 pm apart. The molecule is in the triamino form, rather than one of the possible tautomers.[1]
Thermal decomposition
When heated above 560°, melem transforms into a graphite-like C−N material.[1]
Melemium cations
Melem accepts up to three protons yielding cations called melemium [(NH
2)
3(C
6N
7H
x)]x+
. Some salts described in the literature are melemium sulfate, [(NH2)3(C6N7H2)]SO4 • 2H2O, melemium perchlorate, [(NH2)3(C6N7H)]ClO4 • H2O, melemium hydrogensulfate [(NH2)3(C6N7H3)](HSO4)3 and two melemium methylsulfonates [(NH2)3(C6N7H2)](SO3CH3)2 • H2O and [(NH2)3(C6N7H)][(NH2)3(C6N7H2)](SO3CH3)3 • H2O. The protons can be inserted in any of the six outer nitrogen atoms of the heptazine core, yielding many tautomers of apparently similar energies.[3]
See also
- Triazine H3C3N3, with a single C-N ring
- Melamine (NH2)3(C3N3), triamino triazine
- Melaminium [H(NH2)3(C3N3)]+, a cation derived from melamine
- Melam ((NH2)2(C3N3))2NH, a condensation dimer of melamine
- Melamium [H((NH2)2(C3N3))2NH]+, a cation derived from melam
- Melon (NH2)(NH(C6N7H)NH)nH, a condensation oligomer of melem
References
- ↑ 1.0 1.1 1.2 1.3 Jürgens, Barbara; Irran, Elisabeth; Senker, Jürgen; Kroll, Peter; Müller, Helen; Schnick, Wolfgang (2003-08-01). "Melem (2,5,8-Triamino-tri- s -triazine), an Important Intermediate during Condensation of Melamine Rings to Graphitic Carbon Nitride: Synthesis, Structure Determination by X-ray Powder Diffractometry, Solid-State NMR, and Theoretical Studies" (in en). Journal of the American Chemical Society 125 (34): 10288–10300. doi:10.1021/ja0357689. ISSN 0002-7863. https://pubs.acs.org/doi/10.1021/ja0357689.
- ↑ Tamikuni Komatsu (2001)> Komatsu, Tamikuni (2001-01-01). "The First Synthesis and Characterization of Cyameluric High Polymers" (in en). Macromolecular Chemistry and Physics 202 (1): 19–25. doi:10.1002/1521-3935(20010101)202:1<19::AID-MACP19>3.0.CO;2-G. ISSN 1022-1352. https://onlinelibrary.wiley.com/doi/10.1002/1521-3935(20010101)202:13.0.CO;2-G.
- ↑ Fabian Karl Keßler (2019), Structure and Reactivity of s-Triazine-Based Compounds in C/N/H Chemistry. Doctoral thesis, Fakultät für Chemie und Pharmazie, Ludwig-Maximilians-Universität München
 |
