Chemistry:Methanedisulfonic acid
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
Methanedisulfonic acid | |
| Other names
methionic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| CH4O6S2 | |
| Molar mass | 176.16 g·mol−1 |
| Appearance | colourless solid |
| Melting point | 138–140 °C (280–284 °F; 411–413 K)[1] decomposes: 209-210 °C[2] |
| miscible | |
| Acidity (pKa) | -0.71 (predicted) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H314, H413 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
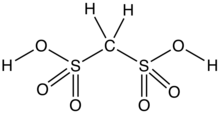
Methanedisulfonic acid is the organosulfur compound with the formula CH2(SO3H)2. It is the disulfonic acid of methane. It is prepared by treatment of methanesulfonic acid with oleum. Its acid strength (pKa) is comparable to that of sulfuric acid.[3]
See also
References
- ↑ Goldwhite, H.; Gibson, M.S.; Harris, C. (January 1965). "Free radical addition reactions—IV". Tetrahedron 21 (10): 2743–2747. doi:10.1016/S0040-4020(01)98360-7.
- ↑ Swan, G. A.; Satchell, D. P. N.; Sykes, K. W.; Michelson, A. M.; Boyd, A. N.; Southern, P. F.; Waters, William A.; Cummings, W. A. W. et al. (1958). "Notes". Journal of the Chemical Society (Resumed): 2051-2068. doi:10.1039/JR9580002051. See note at pages 2058-2060; Cummings, W. A. W. "Some New Sulphur-containing Diacids".
- ↑ Kosswig, Kurt. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a25_503.
 |

