Chemistry:Methoxymethylenetriphenylphosphorane
| |
| Names | |
|---|---|
| IUPAC name
Methoxymethylidene(triphenyl)-λ5-phosphane
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| C20H19OP | |
| Molar mass | 306.345 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
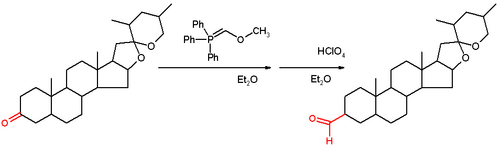
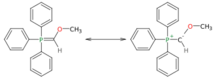
Methoxymethylenetriphenylphosphine is a Wittig reagent used for the homologization of aldehydes, and ketones to extended aldehydes, a organic reaction first reported in 1958.[1] The reagent is generally prepared and used in situ. It has blood-red color, indicative of destabilized ylides.
Preparation
The reagent can be prepared in two steps from triphenylphosphine. The first step is P-alkylation with chloromethyl methyl ether.
- PPh
3 + CH
3OCH
2Cl → [CH
3OCH
2PPh
3]Cl
In the second step, the resulting phosphonium salt is deprotonated.
- [CH
3OCH
2PPh
3]Cl + LiNR
2 → CH
3OCH=PPh
3 + LiCl + HNR
2
In place of chloromethyl methyl ether, a mixture of methylal and acetyl chloride can be used.
Uses
This reagent reacts with a ketone or aldehyde in a Wittig reaction to give an enol ether, which can be converted to the aldehyde by acid-induced hydrolysis.
The initial report of the reaction demonstrated its use on the steroid tigogenone.[2]
It was later used in the Wender Taxol total synthesis and the Stork quinine total synthesis.
References
- ^ A new aldehyde synthesis Samuel G. Levine J. Am. Chem. Soc.; 1958; 80(22); 6150–6151. doi:10.1021/ja01555a068
 |