Chemistry:Methyl ethyl ketone peroxide
| |
| Names | |
|---|---|
| Preferred IUPAC name
2,2′-Peroxydi(butane-2-peroxol) | |
| Other names
2-[(2-Hydroperoxybutan-2-yl)peroxy]butane-2-peroxol
2-Hydroperoxy-2-[(2-hydroperoxybutan-2-yl)peroxy]butane Ketonox Mepox Thermacure | |
| Identifiers | |
3D model (JSmol)
|
|
| 1759757 | |
| ChemSpider | |
| EC Number |
|
| MeSH | Methyl+ethyl+ketone+peroxide |
PubChem CID
|
|
| UNII | |
| UN number | 3105 |
| |
| |
| Properties | |
| C8H18O6 | |
| Molar mass | 210.226 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.170 g cm−3 |
| Boiling point | Decomposition beyond 80 °C (176 °F) [2] |
| Soluble[1] | |
| Explosive data | |
| Shock sensitivity | High |
| Detonation velocity | 5200 m/s |
| RE factor | 0.9 |
| Hazards | |
| Main hazards | Explosive, Toxic |
| GHS pictograms |   
|
| GHS Signal word | DANGER |
| H202, H205, H241, H300, H315, H318, H335 | |
| P102, P220, P243, P250, P261, P264, P280, P283, P370+380, P372, P404 | |
| NFPA 704 (fire diamond) | |
| Flash point | 75 °C (167 °F; 348 K)[2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
none[1] |
REL (Recommended)
|
C 0.2 ppm (1.5 mg/m3)[1] |
IDLH (Immediate danger)
|
N.D.[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
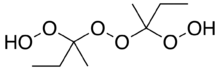
Methyl ethyl ketone peroxide (MEKP) is an organic peroxide with the formula [(CH3)(C2H5)C(O2H)]2O2. MEKP is a colorless oily liquid. It is widely used in vulcanization (crosslinking) of polymers.[3]
It is derived from the reaction of methyl ethyl ketone and hydrogen peroxide under acidic conditions. Several products result from this reaction including a cyclic dimer.[4] The linear dimer, the topic of this article, is the most prevalent.[5] and this is the form that is typically quoted in the commercially available material.[6]
Solutions of 30 to 40% MEKP are used in industry and by hobbyists as catalyst to initiate the crosslinking of unsaturated polyester resins used in fiberglass, and casting. For this application, MEKP often is dissolved in a phlegmatizer such as dimethyl phthalate, cyclohexane peroxide,[clarification needed] or diallyl phthalate (de) to reduce sensitivity to shock. Benzoyl peroxide can be used for the same purpose.[citation needed]
Safety
Whereas acetone peroxide is a white powder at STP, MEKP is slightly less sensitive to shock and temperature, and more stable in storage.
MEKP is a severe skin irritant and can cause progressive corrosive damage or blindness.
The volatile decomposition products of MEKP can contribute to the formation of vapor-phase explosions. Ensuring safe storage is important, and the maximum storage temperature should be limited to below 30 °C.[7]
Notes
- ↑ 1.0 1.1 1.2 1.3 NIOSH Pocket Guide to Chemical Hazards. "#0416". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0416.html.
- ↑ 2.0 2.1 Record of 2-Butanone peroxide in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 10 March 2013.
- ↑ Klenk, Herbert; Götz, Peter H.; Siegmeier, Rainer; Mayr, Wilfried (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_199.
- ↑ Pastureau, P. (1907). "Le superoxyde de la méthyléthylcétone". Comptes Rendus 144 (2): 90–93. https://books.google.com/books?id=zloDAAAAYAAJ&pg=PA90.
- ↑ Milas, N. A.; Golubović, A. (1959). "Studies in Organic Peroxides. XXV. Preparation, Separation and Identification of Peroxides Derived from Methyl Ethyl Ketone and Hydrogen Peroxide". Journal of the American Chemical Society 81 (21): 5824–5826. doi:10.1021/ja01530a068.
- ↑ "2-Butanone peroxide". Sigma-Aldrich. http://www.sigmaaldrich.com/catalog/Lookup.do?N5=All&N3=mode+matchpartialmax&N4=2-Butanone+peroxide&D7=0&D10=2-Butanone+peroxide&N1=S_ID&ST=RS&N25=0&F=PR. Retrieved 2011-12-05.
- ↑ "Methyl Ethyl Ketone Peroxide (MEKP): Production And Uses" (in en-US). 2023-05-19. https://chemcess.com/methyl-ethyl-ketone-peroxide-mekp/.
External links
- CDC - NIOSH Pocket Guide to Chemical Hazards
- The Register: Mass murder in the skies: was the plot feasible?
- New York Times: Details Emerge in British Terror Case
- The Free Information Society: HMTD Synthesis
- How MEKP cures Unsaturated Polyester Resin (video animation)
 |


