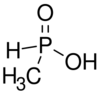
Chemistry:Methylphosphinic acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
Methylphosphinic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| DrugBank | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| CH5O2P | |
| Molar mass | 80.023 g·mol−1 |
| Related compounds | |
Related compounds
|
dimethylphosphinic acid methylphosphonic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Methylphosphinic acid is a monobasic acid, the simplest of the phosphinic acids. A central phosphorus atom is connected to a hydroxy group, a hydrogen atom, a methyl group and an oxygen. Derivatives of methylphosphinic acid can have the phosphorus connected hydrogen atom replaced by other organic groups. In early days what is now called methylphosphonic acid was also called methylphosphinic acid.[1]
Production
Methylphosphinic acid can be produced by the hydrolysis of dimethyl methylphosphonate, which is conveniently obtained from trimethylphosphite.[2]
Hydrolysis of methyldichlorophosphine yields methylphosphinic acid,[3]
Methylphosphinic acid is a common byproduct of the hydrolysis of various CH3P-containing esters and amides, as occur in pesticides for example.[4][5]
Properties
Rat oral -1">50 value for methylphosphinic acid is 940 mg/kg.[4]
Derivatives
- (1,2,5,6-Tetrahydropyridin-4-yl)methylphosphinic acid TPMPA
- N-methylaminomethane-P-methylphosphinic acid[6] or N-methylamino-methyl-methylphosphinic acid[7]
- Ethane-1,2-diylbis(methylphosphinic acid)[8]
- (3-amino-2-hydroxypropyl)methylphosphinic acid
- Methylphosphinic acid ethyl ester (CAS number 16391-07-4)
- dimethylphosphoryloxy-methylphosphinic acid
- N,N-dimethylaminomethane-P-methylphosphinic acid[6]
- (Aminomethyl)methylphosphinic acid 15901-11-8
- (2-aminobenzyl)-methylphosphinic acid[9]
- (6-amino-3-ethyl-1H-benzimidazol-3-ium-2-yl)-methylphosphinic acid
- [(2S,3S)-3-(methoxymethyl)pentan-2-yl]oxy-methylphosphinic acid
- (3-Aminocyclopentyl)methylphosphinic acid[10]
- methylene-di(methylphosphinic acid)
References
- ↑ A. W. Hofman (1872). "New Researches on the Phosphorus Bases" (in en). The Chemical News and Journal of Industrial Science: 245–246. https://books.google.com/books?id=kDBCAQAAMAAJ&pg=PA246.
- ↑ Crofts, Peter C.; Kosolapoff, Gennady M. (1953). "Preparation and Determination of Apparent Dissociation Constants of Some Alkylphosphonic and Dialkylphosphinic Acids1". Journal of the American Chemical Society 75 (14): 3379–3383. doi:10.1021/ja01110a024.
- ↑ Geissbühler, H.; Brooks, G. T.; Kearney, P. C. (2013) (in en). Synthesis of Pesticides Chemical Structure and Biological Activity Natural Products with Biological Activity: Symposia Papers Presented at the Fourth International Congress of Pesticide Chemistry, Zurich, Switzerland, July 24-28, 1978. Elsevier. p. 114. ISBN 9781483278513. https://books.google.com/books?id=Hi4XBQAAQBAJ&pg=PA114.
- ↑ Jump up to: 4.0 4.1 Watson, Rebecca E.; Hafez, Ahmed M.; Kremsky, Jonathan N.; Bizzigotti, George O. (December 2016). "Toxicity of Binary Chemical Munition Destruction Products: Methylphosphonic Acid, Methylphosphinic Acid, 2-Diisopropylaminoethanol, DF Neutralent, and QL Neutralent". International Journal of Toxicology 26 (6): 503–512. doi:10.1080/10915810701707551. PMID 18066966.
- ↑ "Process for the preparation of halo-methylphosphinic acid halides". 30 November 1973. https://patents.google.com/patent/US3943170.
- ↑ Jump up to: 6.0 6.1 Grela, Ewa; Dziełak, Anna; Szydłowska, Katarzyna; Mucha, Artur; Kafarski, Paweł; Grabowiecka, Agnieszka Monika (18 October 2016). "Whole-cell Proteus mirabilis urease inhibition by aminophosphinates for the control of struvite formation". Journal of Medical Microbiology 65 (10): 1123–1129. doi:10.1099/jmm.0.000342. PMID 27550502.
- ↑ Maier, Ludwig (October 1991). "Organic Phosphorus Compounds 98.1Synthesis and Properties of N-Methylaminomethylphosphonic Acid and Derivatives". Phosphorus, Sulfur, and Silicon and the Related Elements 62 (1–4): 29–34. doi:10.1080/10426509108034455.
- ↑ Reiss, Guido J.; Engel, Judith S. (9 January 2008). "Ethane-1,2-diylbis(methylphosphinic acid)". Acta Crystallographica Section E 64 (2): o400. doi:10.1107/S1600536807068122. PMID 21201428. Bibcode: 2008AcCrE..64O.400R.
- ↑ Collins, DJ; Drygala, PF; Swan, JM (1983). "Organophosphorus compounds. XIX. Synthesis of 2,3-Dihydro-1 H-1,2-benzazaphosphole 2-oxides, variously substituted on nitrogen and phosphorus, by N-P cyclization of zwitterionic intermediates". Australian Journal of Chemistry 36 (12): 2517. doi:10.1071/CH9832517.
- ↑ Chebib, Mary; Hanrahan, Jane R.; Kumar, Rohan J.; Mewett, Kenneth N.; Morriss, Gwendolyn; Wooller, Soraya; Johnston, Graham A.R. (March 2007). "(3-Aminocyclopentyl)methylphosphinic acids: Novel GABAC receptor antagonists". Neuropharmacology 52 (3): 779–787. doi:10.1016/j.neuropharm.2006.09.014. PMID 17098260.
 |

