Chemistry:Methylphosphonic acid
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
| MeSH | C032627 |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| CH5O3P | |
| Molar mass | 96.02 |
| Appearance | White Solid |
| Melting point | 105 to 107 °C (221 to 225 °F; 378 to 380 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
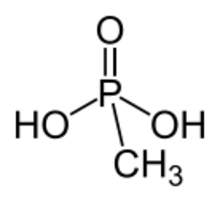
Methylphosphonic acid is an organophosphorus compound with the chemical formula CH3P(O)(OH)2. The phosphorus center is tetrahedral and is bonded to a methyl group, two OH groups and an oxygen. Methylphosphonic acid is a white, non-volatile solid that is poorly soluble in organic solvent but soluble in water and common alcohols.[2]
Preparation
Methylphosphonic acid can be prepared from triethylphosphite by first using a Michaelis-Arbuzov reaction to generate the phosphorus(V) centre:[3]
- CH3Cl + P(OC2H5)3 → CH3PO(OC2H5)2 + C2H5Cl
The resulting dialkylphosphonate is then treated with chlorotrimethylsilane before hydrolysis of the siloxyphosphonate to generate the desired product.[3]
- CH3PO(OC2H5)2 + 2 Me3SiCl → CH3PO(OSiMe3)2 + 2 C2H5Cl
- CH3PO(OSiMe3)2 + 2H2O → CH3PO(OH)2 + 2 HOSiMe3
The reaction pathway proceeds via the siloxyphosphonate intermediate due to the difficulty in directly hydrolysing dialkylphosphonates. Katritzky and co-workers published a one-pot synthesis of methylphosphonic acid in 1989.[3]
References
- ↑ "Methylphosphonic Acid". Sigma-Aldrich. http://www.sigmaaldrich.com/catalog/product/aldrich/289868?lang=en®ion=US. Retrieved 12 December 2013.
- ↑ "methylphosphonic acid - Compound Summary". NCBI. https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=13818. Retrieved 12 December 2013.
- ↑ 3.0 3.1 3.2 Katritzky, Alan R.; Pilarski, Boguslaw; Johnson, Jack W. (1989). "A One-Pot Procedure For the Preparation of Phosphonic Acids From Alkyl Halides". The New Journal for Organic Synthesis 22 (2): 209–213. doi:10.1080/00304949009458197.
 |
