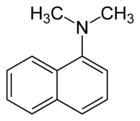
Chemistry:N,N-Dimethyl-1-naphthylamine
From HandWiki
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
N,N-Dimethylnaphthalen-1-amine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties[1][2] | |
| C12H13N | |
| Molar mass | 171.243 g·mol−1 |
| Density | 1.042 g/cm3 at 25 °C |
| Boiling point | 139 to 140 °C (282 to 284 °F; 412 to 413 K) at 13 mmHg |
| Related compounds | |
Related compounds
|
1-naphthylamine 1-naphthol naphthalene aniline dimethylaniline Proton Sponge |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
N,N-Dimethyl-1-naphthylamine is an aromatic amine. It is formally derived from 1-naphthylamine by replacing the hydrogen atoms on the amino group with methyl groups. N,N-Dimethyl-1-naphthylamine is used in the nitrate reductase test to form a precipitate of a red azo dye by reacting with a nitrite-sulfanilic acid complex.[3]
References
- ↑ "N,N-dimethylnaphthalen-1-amine". ChemSpider. http://www.chemspider.com/Chemical-Structure.6587.html. Retrieved 15 April 2010.
- ↑ "D4011 N,N-Dimethyl-1-naphthylamine, ≥98.0% (GC)". Sigma-Aldrich. http://www.sigmaaldrich.com/catalog/product/sial/d4011. Retrieved 15 April 2010.
- ↑ "73426 (Fluka) Nitrate Reduction Test". Sigma-Aldrich. http://www.sigmaaldrich.com/catalog/product/fluka/73426. Retrieved 15 April 2010.
 |

