Chemistry:Sulfanilic acid
| |
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
4-Aminobenzene-1-sulfonic acid[1] | |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H7NO3S | |
| Molar mass | 173.19 |
| Density | 1.485 |
| Melting point | 288 °C (550 °F; 561 K) |
| 12.51 g/L | |
| Related compounds | |
Related sulfonic acids
|
Benzenesulfonic acid p-Toluenesulfonic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
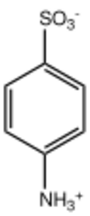
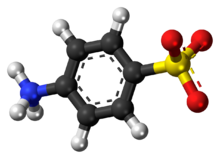
Sulfanilic acid (4-aminobenzenesulfonic acid) is an organic compound with the formula H3NC6H4SO3. It is an off-white solid. It is a zwitterion, which explains its high melting point. It is a common building block in organic chemistry.[2]
Synthesis
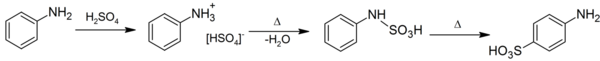
Sulfanilic acid can be produced the sulfonation of aniline with concentrated sulfuric acid.[3] This proceeds via phenylsulfamic acid; a zwitterion with a N-S bond. Eugen Bamberger originally proposed a mechanism involving a series of intramolecular rearrangements, with phenylsulfamic acid forming orthanilic acid, which rearranged to sulfanilic acid on heating.[4][5] Subsequent radiosulphur studies showed that the process is intermolecular, with the phenylsulfamic acid desulfating to generate sulfur trioxide, which then reacts with aniline at the para position in manner similar to a Bamberger rearrangement.[6][7]
Applications
As the compound readily forms diazo compounds, it is used to make dyes and sulfa drugs.[2] This property is also used for the quantitative analysis of nitrate and nitrite ions by diazonium coupling reaction with N-(1-Naphthyl)ethylenediamine, resulting in an azo dye, and the concentration of nitrate or nitrite ions were deduced from the color intensity of the resulting red solution by colorimetry.[8]
It is also used as a standard in combustion analysis and in the Pauly reaction.
Environmental aspects
Reflecting its wide use, sulfanilic acid is found in the leachates of landfills.[9] It is produced by reduction of some azo dyes.[10]
Derivatives
- Methyl orange (azo coupling with dimethylaniline)
- Acid orange 7 (azo coupling with 2-naphthol)[11]
- Chrysoine resorcinol (azo coupling with resorcinol)
See also
References
- ↑ 1.0 1.1 Favre, Henri A., ed (2014). Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: Royal Society of Chemistry. p. 789. doi:10.1039/9781849733069-FP001. ISBN 9780854041824. OCLC 1077224056. https://books.google.com/books?id=4USgAgAAQBAJ&pg=PA789. "The name ‘sulfanilic acid’ is not retained."
- ↑ 2.0 2.1 "Sulphanilic acid". A Dictionary of Chemistry. Oxford University Press, 2000. Oxford Reference Online. Oxford University Press.
- ↑ Siegfried Hauptmann: Organische Chemie, 2nd Edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, p. 511, ISBN:3-342-00280-8.
- ↑ Bamberger, Eug.; Kunz, Jac. (May 1897). "Umlagerung von Sulfonsäuren. (II.)". Berichte der Deutschen Chemischen Gesellschaft 30 (2): 2274–2277. doi:10.1002/cber.189703002211. https://zenodo.org/record/1773854.
- ↑ Illuminati, Gabriello (June 1956). "A Reinvestigation of the Role of Phenylsulfamic Acid in the Formation of Aminobenzenesulfonic Acids". Journal of the American Chemical Society 78 (11): 2603–2606. doi:10.1021/ja01592a075.
- ↑ Spillane, W.J.; Scott, F.L. (January 1967). "Radiosulphur studies on the rearrangement of phenylsulphamic acid to sulphanilic acid". Tetrahedron Letters 8 (14): 1251–1253. doi:10.1016/S0040-4039(00)90678-6.
- ↑ Scott, F.L.; Spillane, W.J. (January 1967). "The isomerization of orthanilic acid to sulphanilic acid in the presence of sulphuric acid-S35". Tetrahedron Letters 8 (28): 2707–2711. doi:10.1016/S0040-4039(01)89979-2.
- ↑ G. H. Jerffery; J. Bassett; J. Mendham; R. C. Denney (1989). "Colorimetry and Spectrophotometry". Vogel's Textbook of Quantitative Chemical Analysis, 5th Edition. Longman. p. 702. ISBN 0-582-44693-7.
- ↑ Holm, John V.; Ruegge, Kirsten.; Bjerg, Poul L.; Christensen, Thomas H. (1995). "Occurrence and Distribution of Pharmaceutical Organic Compounds in the Groundwater Downgradient of a Landfill (Grindsted, Denmark)". Environmental Science & Technology 29 (5): 1415–1420. doi:10.1021/es00005a039. PMID 22192041.
- ↑ Nam, S. (2000). "Reduction of azo dyes with zero-valent iron". Water Research 34 (6): 1837–1845. doi:10.1016/S0043-1354(99)00331-0.
- ↑ Klaus Hunger; Peter Mischke; Wolfgang Rieper; Roderich Raue; Klaus Kunde; Aloys Engel (2005). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_245..
 |