Chemistry:N-Nitrosodiphenylamine
From HandWiki
Short description: Industrial compound
| |
| Names | |
|---|---|
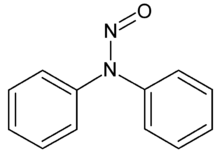
| IUPAC name
N,N-diphenylnitrous amide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3077 3082 |
| |
| |
| Properties | |
| C12H10N2O | |
| Molar mass | 198.225 g·mol−1 |
| Density | 1.23 |
| Melting point | 66.5 °C (151.7 °F; 339.6 K) |
| Boiling point | 101 °C (214 °F; 374 K) |
| Structure[1] | |
| Monoclinic | |
| C2/c | |
a = 16.28, b = 8.827, c = 16.51 α = 90°, β = 117.53°, γ = 90° Å
| |
Lattice volume (V)
|
2104.0 |
Formula units (Z)
|
8 |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Warning |
| H302, H315, H317, H319, H351, H361, H373, H410, H411 | |
| P201, P202, P260, P261, P264, P270, P272, P273, P280, P281, P301+312, P302+352, P305+351+338, P308+313, P314, P321, P330, P332+313, P333+313, P337+313, P362, P363, P391, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
N-Nitrosodiphenylamine is an industrial compound which is commonly used to create rubber products.[2]
References
- ↑ Banerjee, A.; Brown, C. J.; Lewis, J. F. P. (15 October 1982). "N-Nitrosodiphenylamine". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry 38 (10): 2744–2745. doi:10.1107/S0567740882009832.
- ↑ "ATSDR - Toxic Substances - n-Nitrosodiphenylamine". https://www.atsdr.cdc.gov/substances/toxsubstance.asp?toxid=212.

