Chemistry:Nicotinamide mononucleotide
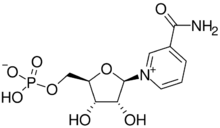
| |
| Names | |
|---|---|
| IUPAC name
3-Carbamoyl-1-(5-O-phosphono-β-D-ribofuranosyl)pyridin-1-ium
| |
| Systematic IUPAC name
[(2R,3S,4R,5R)-5-(3-Carbamoylpyridin-1-ium-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl hydrogen phosphate | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3570187 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C11H15N2O8P | |
| Molar mass | 334.221 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nicotinamide mononucleotide ("NMN" and "β-NMN") is a nucleotide derived from ribose, nicotinamide, nicotinamide riboside and niacin.[1] In humans, several enzymes use NMN to generate nicotinamide adenine dinucleotide (NADH).[1] In mice, it has been proposed that NMN is absorbed via the small intestine within 10 minutes of oral uptake and converted to nicotinamide adenine dinucleotide (NAD+) through the Slc12a8 transporter.[2] However, this observation has been challenged,[3] and the matter remains unsettled.[4]
Because NADH is a cofactor for processes inside mitochondria, for sirtuins and PARP, NMN has been studied in animal models as a potential neuroprotective and anti-aging agent.[5][6] The reversal of aging at the cellular level by inhibiting mitochondrial decay in presence of increased levels of NAD+ makes it popular among anti-aging products.[7] Dietary supplement companies have aggressively marketed NMN products, claiming those benefits.[8] However, no human studies to date have properly proven its anti-aging effects. Single-dose administration of up to 500 mg was shown safe in men in a study at Keio University.[9] One 2021 clinical trial found that NMN improved muscular insulin sensitivity in prediabetic women,[10] while another found that it improved aerobic capacity in amateur runners.[11] A 2023 clinical trial showed that NMN improves performance on a six-minute walking test and a subjective general health assessment.[12]
NMN is vulnerable to extracellular degradation by CD38 enzyme,[13] which can be inhibited by compounds such as CD38-IN-78c.[14]
Dietary sources
NMN is found in fruits and vegetables such as edamame, broccoli, cabbage, cucumber and avocado at a concentration of about 1 mg per 100g,[15][16][17] making these natural sources impractical to acquire the quantities needed to accomplish the dosing currently being investigated for NMN as a pharmaceutical.
Production
Production of nicotinamide mononucleotide has been redacted since the latter half of 2022 by the FDA because it is under investigation as a pharmaceutical drug.[18][19]
Different expressions of NMN across human organs
The synthesizing enzymes and consumption enzymes of NMN also exhibit tissue specificity: NMN is widely distributed in tissues and organs throughout the body and has been present in various cells since embryonic development.[19]
Potential benefits and risks
NMN is a precursor for NAD+ biosynthesis, and NMN dietary supplementation has been demonstrated to increase NAD+ concentration and thus has the potential to mitigate aging-related disorders such as oxidative stress, DNA damage, neurodegeneration and inflammatory responses.[20] The potential benefits and risks of NMN supplementation, as of 2023, are currently under investigation.[20]
Certain enzymes are sensitive to the intracellular NMN/NAD+ ratio, such as SARM1,[21] a protein responsible for initiating cellular degeneration pathways such as MAP kinase and inducing axonal loss and neuronal death.[22][23] NMNAT is an enzyme with neurorescuing properties that functions to deplete NMN and produce NAD+, attenuating SARM1 activity and aiding neuronal survival in-vitro,[24][25] an effect that is reversed by applying exogenous NMN which promptly resumed axon destruction.[26] The similar molecule nicotinic acid mononucleotide (NaMN) opposes the activating effect of NMN on SARM1, and is a neuroprotector.[27]
References
- ↑ 1.0 1.1 Roger Lee, Roger (2023). "Different Expressions of NMN Across Human Organs". American Journal of Sociology. https://www.nmnsupplier.com/what-is-nmn.html.
- ↑ Grozio, A; Mills, KF; Yoshino, J; Bruzzone, S; Sociali, G; Tokizane, K; Lei, HC; Cunningham, R et al. (January 2019). "Slc12a8 is a nicotinamide mononucleotide transporter.". Nature Metabolism 1 (1): 47–57. doi:10.1038/s42255-018-0009-4. PMID 31131364.
- ↑ Schmidt, MS; Brenner, C (July 2019). "Absence of evidence that Slc12a8 encodes a nicotinamide mononucleotide transporter.". Nature Metabolism 1 (7): 660–661. doi:10.1038/s42255-019-0085-0. PMID 32694648.
- ↑ Chini, CCS; Zeidler, JD; Kashyap, S; Warner, G; Chini, EN (1 June 2021). "Evolving concepts in NAD+ metabolism.". Cell Metabolism 33 (6): 1076–1087. doi:10.1016/j.cmet.2021.04.003. PMID 33930322.
- ↑ "+ synthase… It's a chaperone… It's a neuroprotector". Current Opinion in Genetics & Development 44: 156–162. June 2017. doi:10.1016/j.gde.2017.03.014. PMID 28445802.
- ↑ Mills, Kathryn F.; Yoshida, Shohei; Stein, Liana R.; Grozio, Alessia; Kubota, Shunsuke; Sasaki, Yo; Redpath, Philip; Migaud, Marie E. et al. (13 December 2016). "Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice". Cell Metabolism 24 (6): 795–806. doi:10.1016/j.cmet.2016.09.013. PMID 28068222.
- ↑ Nadeeshani, Harshani; Li, Jinyao; Ying, Tianlei; Zhang, Baohong; Lu, Jun (1 March 2022). "Nicotinamide mononucleotide (NMN) as an anti-aging health product – Promises and safety concerns" (in en). Journal of Advanced Research 37: 267–278. doi:10.1016/j.jare.2021.08.003. ISSN 2090-1232. PMID 35499054.
- ↑ "Beyond Resveratrol: The Anti-Aging NAD Fad" (in en). Scientific American Blog Network. March 11, 2015. https://blogs.scientificamerican.com/guest-blog/beyond-resveratrol-the-anti-aging-nad-fad.
- ↑ Irie, Junichiro; Inagaki, Emi; Fujita, Masataka; Nakaya, Hideaki; Mitsuishi, Masanori; Yamaguchi, Shintaro; Yamashita, Kazuya; Shigaki, Shuhei et al. (2020). "Effect of oral administration of nicotinamide mononucleotide on clinical parameters and nicotinamide metabolite levels in healthy Japanese men" (in en). Endocrine Journal 67 (2): 153–60. doi:10.1507/endocrj.EJ19-0313. ISSN 0918-8959. PMID 31685720. https://www.jstage.jst.go.jp/article/endocrj/67/2/67_EJ19-0313/_article.
- ↑ "Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women". Science 372 (6547): 1224–29. June 2021. doi:10.1126/science.abe9985. PMID 33888596.
- ↑ Liao, B; Zhao, Y; Wang, D; Zhang, X; Hao, X; Hu, M (2021). ""Nicotinamide mononucleotide supplementation enhances aerobic capacity in amateur runners: a randomized, double-blind study"". Journal of the International Society of Sports Nutrition 18 (1): 54. doi:10.1186/s12970-021-00442-4. PMID 34238308.
- ↑ Yi Lin (Feb 2023). "The efficacy and safety of β-nicotinamide mononucleotide (NMN) supplementation in healthy middle-aged adults: a randomized, multicenter, double-blind, placebo-controlled, parallel-group, dose-dependent clinical trial". Geroscience 45 (1): 29–43. doi:10.1007/s11357-022-00705-1. PMID 36482258.
- ↑ "+ Synthesis and Functions in Mammalian Cells". Trends in Biochemical Sciences 45 (10): 858–73. October 2020. doi:10.1016/j.tibs.2020.05.010. PMID 32595066. PMC 7502477. https://www.jbc.org/content/294/52/19831.long.
- ↑ "A Potent and Specific CD38 Inhibitor Ameliorates Age-Related Metabolic Dysfunction by Reversing Tissue NAD+ Decline". Cell Metab 27 (5): 1081–95.e10. May 2018. doi:10.1016/j.cmet.2018.03.016. PMID 29719225.
- ↑ Mills, KF; Yoshida, S; Stein, LR; Grozio, A; Kubota, S; Sasaki, Y; Redpath, P; Migaud, ME et al. (13 December 2016). "Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice.". Cell Metabolism 24 (6): 795–806. doi:10.1016/j.cmet.2016.09.013. PMID 28068222.
- ↑ Ryan, Finn (2016-12-06). "5 Anti-Aging Food Types You Should Already Be Eating" (in en-US). https://www.bicycling.com/health-nutrition/g20011748/5-anti-aging-food-types-you-should-already-be-eating/.
- ↑ "Scientists identify new fuel-delivery route for cells" (in en). 2019-01-07. https://medicine.wustl.edu/news/scientists-identify-new-fuel-delivery-route-for-cells/.
- ↑ nutraingredients-usa.com/Article/2023/02/16/Amazon-removing-NMN-dietary-supplements-citing-FDA-actions
- ↑ 19.0 19.1 https://www.nmn.com/news/fda-bans-labeling-nmn-as-a-supplement
- ↑ 20.0 20.1 Song Q, Zhou X, Xu K, Liu S, Zhu X, Yang J. The Safety and Antiaging Effects of Nicotinamide Mononucleotide in Human Clinical Trials: an Update. Adv Nutr. 2023 Nov;14(6):1416-1435. doi: 10.1016/j.advnut.2023.08.008. Epub 2023 Aug 22. PMID: 37619764; PMCID: PMC10721522
- ↑ Figley, Matthew D.; Gu, Weixi; Nanson, Jeffrey D.; Shi, Yun; Sasaki, Yo; Cunnea, Katie; Malde, Alpeshkumar K.; Jia, Xinying et al. (7 April 2021). "SARM1 is a metabolic sensor activated by an increased NMN/NAD+ ratio to trigger axon degeneration". Neuron 109 (7): 1118–1136.e11. doi:10.1016/j.neuron.2021.02.009. ISSN 1097-4199. PMC 8174188. https://pubmed.ncbi.nlm.nih.gov/33657413.
- ↑ Di Stefano, M; Nascimento-Ferreira, I; Orsomando, G; Mori, V; Gilley, J; Brown, R; Janeckova, L; Vargas, M E et al. (April 2015). "A rise in NAD precursor nicotinamide mononucleotide (NMN) after injury promotes axon degeneration". Cell Death and Differentiation 22 (5): 731–742. doi:10.1038/cdd.2014.164. ISSN 1350-9047. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4392071.
- ↑ Zhao, Zhi Ying; Xie, Xu Jie; Li, Wan Hua; Liu, Jun; Chen, Zhe; Zhang, Ben; Li, Ting; Li, Song Lu et al. (4 May 2019). "A Cell-Permeant Mimetic of NMN Activates SARM1 to Produce Cyclic ADP-Ribose and Induce Non-apoptotic Cell Death". iScience 15: 452–466. doi:10.1016/j.isci.2019.05.001. ISSN 2589-0042. PMC 6531917. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6531917.
- ↑ Brazill, Jennifer M.; Li, Chong; Zhu, Yi; Zhai, R. Grace (26 April 2017). "NMNAT: It’s an NAD+ Synthase… It’s a Chaperone… It’s a Neuroprotector". Current opinion in genetics & development 44: 156–162. doi:10.1016/j.gde.2017.03.014. ISSN 0959-437X. PMC 5515290. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5515290/.
- ↑ Gerdts, Josiah; Summers, Daniel W.; Milbrandt, Jeffrey; DiAntonio, Aaron (3 February 2016). "Axon self destruction: new links among SARM1, MAPKs, and NAD+ metabolism". Neuron 89 (3): 449–460. doi:10.1016/j.neuron.2015.12.023. ISSN 0896-6273. PMC 4742785. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4742785/.
- ↑ Di Stefano, M; Nascimento-Ferreira, I; Orsomando, G; Mori, V; Gilley, J; Brown, R; Janeckova, L; Vargas, M E et al. (April 2015). "A rise in NAD precursor nicotinamide mononucleotide (NMN) after injury promotes axon degeneration". Cell Death and Differentiation 22 (5): 731–742. doi:10.1038/cdd.2014.164. ISSN 1350-9047. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4392071/.
- ↑ Sasaki, Yo; Zhu, Jian; Shi, Yun; Gu, Weixi; Kobe, Bostjan; Ve, Thomas; DiAntonio, Aaron; Milbrandt, Jeffrey (November 2021). "Nicotinic acid mononucleotide is an allosteric SARM1 inhibitor promoting axonal protection". Experimental neurology 345: 113842. doi:10.1016/j.expneurol.2021.113842. ISSN 0014-4886. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8571713.

