Biology:Sirtuin
| Sir2 family | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
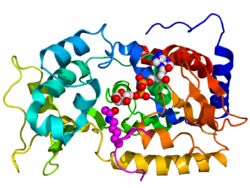 Crystallographic structure of yeast sir2 (rainbow colored cartoon, N-terminus = blue, C-terminus = red) complexed with ADP (space-filling model, carbon = white, oxygen = red, nitrogen = blue, phosphorus = orange) and a histone H4 peptide (magenta) containing an acylated lysine residue (displayed as spheres).[1] | |||||||||||
| Identifiers | |||||||||||
| Symbol | SIR2 | ||||||||||
| Pfam | PF02146 | ||||||||||
| Pfam clan | CL0085 | ||||||||||
| InterPro | IPR003000 | ||||||||||
| PROSITE | PS50305 | ||||||||||
| SCOP2 | 1j8f / SCOPe / SUPFAM | ||||||||||
| |||||||||||
Sirtuins are a family of signaling proteins involved in metabolic regulation.[2][3] They are ancient in animal evolution and appear to possess a highly conserved structure throughout all kingdoms of life.[2] Chemically, sirtuins are a class of proteins that possess either mono-ADP-ribosyltransferase or deacylase activity, including deacetylase, desuccinylase, demalonylase, demyristoylase and depalmitoylase activity.[4][5][6] The name Sir2 comes from the yeast gene 'silent mating-type information regulation 2',[7] the gene responsible for cellular regulation in yeast.
From in vitro studies, sirtuins are implicated in influencing cellular processes like aging, transcription, apoptosis, inflammation[8] and stress resistance, as well as energy efficiency and alertness during low-calorie situations.[9] As of 2018, there was no clinical evidence that sirtuins affect human aging.[10]
Yeast Sir2 and some, but not all, sirtuins are protein deacetylases. Unlike other known protein deacetylases, which simply hydrolyze acetyl-lysine residues, the sirtuin-mediated deacetylation reaction couples lysine deacetylation to NAD+ hydrolysis.[11] This hydrolysis yields O-acetyl-ADP-ribose, the deacetylated substrate and nicotinamide, which is an inhibitor of sirtuin activity itself. These proteins utilize NAD+ to maintain cellular health and turn NAD+ to nicotinamide (NAM).[12] The dependence of sirtuins on NAD+ links their enzymatic activity directly to the energy status of the cell via the cellular NAD+:NADH ratio, the absolute levels of NAD+, NADH or NAM or a combination of these variables.
Sirtuins that deacetylate histones are structurally and mechanistically distinct from other classes of histone deacetylases (classes I, IIA, IIB and IV), which have a different protein fold and use Zn2+ as a cofactor.[13][14]
Actions and species distribution
Sirtuins are a family of signaling proteins involved in metabolic regulation.[2][3] They are ancient in animal evolution and appear to possess a highly conserved structure throughout all kingdoms of life.[2] Whereas bacteria and archaea encode either one or two sirtuins, eukaryotes encode several sirtuins in their genomes. In yeast, roundworms, and fruitflies, sir2 is the name of one of the sirtuin-type proteins (see table below).[15] Mammals possess seven sirtuins (SIRT1–7) that occupy different subcellular compartments: SIRT1, SIRT6 and SIRT7 are predominantly in the nucleus, SIRT2 in the cytoplasm, and SIRT3, SIRT4 and SIRT5 in the mitochondria.[2]
History
Research on sirtuin protein was started in 1991 by Leonard Guarente of MIT.[16][17] Interest in the metabolism of NAD+ heightened after the year 2000 discovery by Shin-ichiro Imai and coworkers in the Guarente laboratory that sirtuins are NAD+-dependent protein deacetylases .[18]
Types
The first sirtuin was identified in yeast (a lower eukaryote) and named sir2. In more complex mammals, there are seven known enzymes that act in cellular regulation, as sir2 does in yeast. These genes are designated as belonging to different classes (I-IV), depending on their amino acid sequence structure.[19] Several gram positive prokaryotes as well as the gram negative hyperthermophilic bacterium Thermotoga maritima possess sirtuins that are intermediate in sequence between classes, and these are placed in the "undifferentiated" or "U" class. In addition, several Gram positive bacteria, including Staphylococcus aureus and Streptococcus pyogenes, as well as several fungi carry macrodomain-linked sirtuins (termed "class M" sirtuins).[6]
| Class | Subclass | Species | Intracellular location |
Activity | Cellular Function | Catalytic Domains[20] | Histone Deacetylation Target[21] | Non-Histone Deacetylation Target[21] | Pathology[21] | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacteria | Yeast | Mouse | Human | |||||||||
| I | a | Sir2, Hst1 |
Sirt1 | SIRT1 | Nucleus, cytoplasm | Deacetylase | Metabolism inflammation | 244-498 (of 766aa) | H3K9ac, H1K26ac, H4K16ac | Hif-1α, Hif-2α, MYC, P53, BRCA1, FOXO3A, MyoD, Ku70, PPARγ, PCAF, Suv39h1, TGFB1, WRN, NBS1 | Neurodegenerative diseases, Cancer: acute myeloid leukemia, colon, prostate, ovarian, glioma, breast, melanoma, lung adenocarcinoma | |
| b | Hst2 | Sirt2 | SIRT2 | Nucleus and cytoplasm | Deacetylase | Cell cycle, tumorigenesis | 65-340 (of 388aa) | H3K56ac, H4K16ac | Tubulin, Foxo3a, EIF5A, P53, G6PD, MYC | Neurodegenerative diseases, Cancer: brain tissue, glioma | ||
| Sirt3 | SIRT3 | Mitochondria | Deacetylase | Metabolism | 126-382 (of 399aa) | H3K56ac, H4K14ac | SOD2, PDH, IDH2, GOT2, FoxO3a | Neurodegenerative diseases, Cancer: B cell chronic lymphocytic leukemia, mantle cell lymphoma, chronic lymphocytic leukemia, breast, gastric | ||||
| c | Hst3, Hst4 |
|||||||||||
| II | Sirt4 | SIRT4 | Mitochondria | ADP-ribosyl transferase | Insulin secretion | 45-314 (of 314aa) | Unknown | GDH, PDH | Cancer: breast, colorectal | |||
| III | Sirt5 | SIRT5 | Mitochondria | Demalonylase, desuccinylase and deacetylase | Ammonia detoxification | 41-309 (of 310aa) | Unknown | CPS1 | Cancer: pancreatic, breast, non-small cell lung carcinoma | |||
| IV | a | Sirt6 | SIRT6 | Nucleus | Demyristoylase, depalmitoylase, ADP-ribosyl transferase and deacetylase | DNA repair, metabolism, TNF secretion | 35-274 (of 355aa) | H3K9ac, H3K56ac | Unknown | Cancer: breast, colon | ||
| b | Sirt7 | SIRT7 | Nucleolus | Deacetylase | rRNA transcription | 90-331 (of 400aa) | H3K18ac | Hif-1α, Hif-2α | Cancer: liver, testis, spleen, thyroid, breast | |||
| U | cobB[22] | Regulation of acetyl-CoA synthetase[23] | metabolism | |||||||||
| M | SirTM[6] | ADP-ribosyl transferase | ROS detoxification | |||||||||
SIRT3, a mitochondrial protein deacetylase, plays a role in the regulation of multiple metabolic proteins like isocitrate dehydrogenase of the TCA cycle. It also plays a role in skeletal muscle as a metabolic adaptive response. Since glutamine is a source of a-ketoglutarate used to replenish the TCA cycle, SIRT4 is involved in glutamine metabolism.[24]
Ageing
Although preliminary studies with resveratrol, an activator of deacetylases such as SIRT1,[25] led some scientists to speculate that resveratrol may extend lifespan, no clinical evidence for such an effect has been discovered, as of 2018.[10]
Tissue fibrosis
A 2018 review indicated that SIRT levels are lower in tissues from people with scleroderma, and such reduced SIRT levels may increase risk of fibrosis through modulation of the TGF-β signaling pathway.[26]
DNA repair in laboratory studies
SIRT1, SIRT6 and SIRT7 proteins are employed in DNA repair.[27] SIRT1 protein promotes homologous recombination in human cells and is involved in recombinational repair of DNA breaks.[28]
SIRT6 is a chromatin-associated protein and in mammalian cells is required for base excision repair of DNA damage.[29] SIRT6 deficiency in mice leads to a degenerative aging-like phenotype.[29] In addition, SIRT6 promotes the repair of DNA double-strand breaks.[30] Furthermore, over-expression of SIRT6 can stimulate homologous recombinational repair.[31]
SIRT7 knockout mice display features of premature aging.[32] SIRT7 protein is required for repair of double-strand breaks by non-homologous end joining.[32]
Inhibitors
Certain sirtuin activity is inhibited by nicotinamide, which binds to a specific receptor site.[33] It is an inhibitor in vitro of SIRT1, but can be a stimulator in cells.[34]
Activators
| Compound | Target/Specificity | References |
|---|---|---|
| Piceatannol | SIRT1 | [35] |
| SRT-1720 | SIRT1 | [35] |
| SRT-2104 | SIRT1 | [35] |
| Beta-Lapachone | SIRT1 | [35] |
| Cilostazol | SIRT1 | [35] |
| Quercetin and rutin derivatives | SIRT6 | [36] |
| Luteolin | SIRT6 | [36] |
| Fisetin | SIRT6 | [36] |
| Phenolic acid | SIRT6 | [36] |
| Fucoidan | SIRT6 | [37] |
| Curcumin | SIRT1, SIRT6 | [38] |
| Pirfenidone | SIRT1 | [39] |
| Myricetin | SIRT6 | [36] |
| Cyanidin | SIRT6 | [36] |
| Delphinidin | SIRT6 | [36] |
| Apigenin | SIRT6 | [36] |
| Butein | SIRT6 | [40] |
| Isoliquiritigenin | SIRT6 | [40] |
| Ferulic acid | SIRT1 | [40] |
| Berberine | SIRT1 | [40] |
| Catechin | SIRT1 | [40] |
| Malvidin | SIRT1 | [40] |
| Pterostilbene | SIRT1 | [40] |
| Tyrosol | SIRT1 | [40] |
See also
References
- ↑ PDB: 1szd; "Structural basis for nicotinamide cleavage and ADP-ribose transfer by NAD(+)-dependent Sir2 histone/protein deacetylases". Proceedings of the National Academy of Sciences of the United States of America 101 (23): 8563–8. June 2004. doi:10.1073/pnas.0401057101. PMID 15150415. Bibcode: 2004PNAS..101.8563Z.
- ↑ 2.0 2.1 2.2 2.3 2.4 Ye, X; Li, M; Hou, T; Gao, T; Zhu, WG; Yang, Y (3 January 2017). "Sirtuins in glucose and lipid metabolism.". Oncotarget 8 (1): 1845–1859. doi:10.18632/oncotarget.12157. PMID 27659520.
- ↑ 3.0 3.1 "Sirtuin functions in health and disease". Molecular Endocrinology 21 (8): 1745–55. August 2007. doi:10.1210/me.2007-0079. PMID 17456799.
- ↑ "Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase". Science 334 (6057): 806–9. November 2011. doi:10.1126/science.1207861. PMID 22076378. Bibcode: 2011Sci...334..806D.
- ↑ "SIRT6 regulates TNF-α secretion through hydrolysis of long-chain fatty acyl lysine". Nature 496 (7443): 110–3. April 2013. doi:10.1038/nature12038. PMID 23552949. Bibcode: 2013Natur.496..110J.
- ↑ 6.0 6.1 6.2 "Identification of a Class of Protein ADP-Ribosylating Sirtuins in Microbial Pathogens". Molecular Cell 59 (2): 309–20. July 2015. doi:10.1016/j.molcel.2015.06.013. PMID 26166706.
- ↑ EntrezGene 23410
- ↑ "Sirtuin deacylases: a molecular link between metabolism and immunity". Journal of Leukocyte Biology 93 (5): 669–80. May 2013. doi:10.1189/jlb.1112557. PMID 23325925.
- ↑ "SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus". The Journal of Neuroscience 30 (30): 10220–32. July 2010. doi:10.1523/JNEUROSCI.1385-10.2010. PMID 20668205.
- ↑ 10.0 10.1 Shetty, Ashok K.; Kodali, Maheedhar; Upadhya, Raghavendra; Madhu, Leelavathi N. (2018). "Emerging anti-aging strategies - scientific basis and efficacy (Review)". Aging and Disease 9 (6): 1165–1184. doi:10.14336/ad.2018.1026. ISSN 2152-5250. PMID 30574426.
- ↑ "Biological and catalytic functions of sirtuin 6 as targets for small-molecule modulators". Journal of Biological Chemistry 295 (32): 11021–11041. 2020. doi:10.1074/jbc.REV120.011438. PMID 32518153.
- ↑ "NMN vs NR: The Differences Between These 2 NAD+ Precursors". https://www.nmn.com/precursors/nmn-vs-nr.
- ↑ "Structural and chemical biology of deacetylases for carbohydrates, proteins, small molecules and histones". Communications Biology 1: 217. 2018. doi:10.1038/s42003-018-0214-4. PMID 30534609.
- ↑ "Histone deacetylase inhibitors: Potential in cancer therapy". Journal of Cellular Biochemistry 107 (4): 600–8. July 2009. doi:10.1002/jcb.22185. PMID 19459166.
- ↑ "The Sir2 family of protein deacetylases". Annual Review of Biochemistry 73 (1): 417–35. 2004. doi:10.1146/annurev.biochem.73.011303.073651. PMID 15189148.
- ↑ Wade N (2006-11-08). "The quest for a way around aging". Health & Science. International Herald Tribune. http://www.iht.com/articles/2006/11/08/healthscience/snwonder.php.
- ↑ "MIT researchers uncover new information about anti-aging gene". Massachusetts Institute of Technology, News Office. 2000-02-16. http://web.mit.edu/newsoffice/2000/guarente.html.
- ↑ "Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase". Nature 403 (6771): 795–800. 2000. doi:10.1038/35001622. PMID 10693811. Bibcode: 2000Natur.403..795I.
- ↑ "Role for human SIRT2 NAD-dependent deacetylase activity in control of mitotic exit in the cell cycle". Molecular and Cellular Biology 23 (9): 3173–85. May 2003. doi:10.1128/MCB.23.9.3173-3185.2003. PMID 12697818.
- ↑ Chang, Andrew R.; Ferrer, Christina M.; Mostoslavsky, Raul (2020-01-01). "SIRT6, a Mammalian Deacylase with Multitasking Abilities" (in en). Physiological Reviews 100 (1): 145–169. doi:10.1152/physrev.00030.2018. PMID 31437090.
- ↑ 21.0 21.1 21.2 Carafa, Vincenzo; Rotili, Dante; Forgione, Mariantonietta; Cuomo, Francesca; Serretiello, Enrica; Hailu, Gebremedhin Solomon; Jarho, Elina; Lahtela-Kakkonen, Maija et al. (2016-05-25). "Sirtuin functions and modulation: from chemistry to the clinic". Clinical Epigenetics 8: 61. doi:10.1186/s13148-016-0224-3. ISSN 1868-7075. PMID 27226812.
- ↑ "Structure and substrate binding properties of cobB, a Sir2 homolog protein deacetylase from Escherichia coli". Journal of Molecular Biology 337 (3): 731–41. March 2004. doi:10.1016/j.jmb.2004.01.060. PMID 15019790.
- ↑ "Conserved metabolic regulatory functions of sirtuins". Cell Metabolism 7 (2): 104–12. February 2008. doi:10.1016/j.cmet.2007.11.006. PMID 18249170.
- ↑ "Sirtuins, metabolism, and DNA repair". Current Opinion in Genetics & Development 26: 24–32. June 2014. doi:10.1016/j.gde.2014.05.005. PMID 25005742.
- ↑ Aunan, JR; Watson, MM; Hagland, HR; Søreide, K (January 2016). "Molecular and biological hallmarks of ageing.". The British Journal of Surgery 103 (2): e29-46. doi:10.1002/bjs.10053. PMID 26771470.
- ↑ "Sirtuins and accelerated aging in scleroderma". Current Rheumatology Reports 20 (4): 16. March 2018. doi:10.1007/s11926-018-0724-6. PMID 29550994.
- ↑ "Sirtuins and DNA damage repair: SIRT7 comes to play". Nucleus 8 (2): 107–115. March 2017. doi:10.1080/19491034.2016.1264552. PMID 28406750.
- ↑ "Role of SIRT1 in homologous recombination". DNA Repair 9 (4): 383–93. April 2010. doi:10.1016/j.dnarep.2009.12.020. PMID 20097625.
- ↑ 29.0 29.1 "Genomic instability and aging-like phenotype in the absence of mammalian SIRT6". Cell 124 (2): 315–29. January 2006. doi:10.1016/j.cell.2005.11.044. PMID 16439206.
- ↑ "SIRT6 stabilizes DNA-dependent protein kinase at chromatin for DNA double-strand break repair". Aging 1 (1): 109–21. January 2009. doi:10.18632/aging.100011. PMID 20157594.
- ↑ "Sirtuin 6 (SIRT6) rescues the decline of homologous recombination repair during replicative senescence". Proceedings of the National Academy of Sciences of the United States of America 109 (29): 11800–5. July 2012. doi:10.1073/pnas.1200583109. PMID 22753495. Bibcode: 2012PNAS..10911800M.
- ↑ 32.0 32.1 "SIRT7 promotes genome integrity and modulates non-homologous end joining DNA repair". The EMBO Journal 35 (14): 1488–503. July 2016. doi:10.15252/embj.201593499. PMID 27225932.
- ↑ "Mechanism of sirtuin inhibition by nicotinamide: altering the NAD(+) cosubstrate specificity of a Sir2 enzyme". Molecular Cell 17 (6): 855–68. March 2005. doi:10.1016/j.molcel.2005.02.022. PMID 15780941.
- ↑ "Nicotinamide is an inhibitor of SIRT1 in vitro, but can be a stimulator in cells". Cell Mol Life Sci 74 (18): 3347–3362. September 2017. doi:10.1007/s00018-017-2527-8. PMID 28417163.
- ↑ 35.0 35.1 35.2 35.3 35.4 Manjula, Ramu; Anuja, Kumari; Alcain, Francisco J. (12 January 2021). "SIRT1 and SIRT2 Activity Control in Neurodegenerative Diseases". Frontiers in Pharmacology 11: 585821. doi:10.3389/fphar.2020.585821. PMID 33597872.
- ↑ 36.0 36.1 36.2 36.3 36.4 36.5 36.6 36.7 Rahnasto-Rilla, Minna; Tyni, Jonna; Huovinen, Marjo et al. (7 March 2018). "Natural polyphenols as sirtuin 6 modulators" (in en). Scientific Reports 8 (1): 4163. doi:10.1038/s41598-018-22388-5. PMID 29515203. Bibcode: 2018NatSR...8.4163R.
- ↑ Rahnasto-Rilla, Minna K.; McLoughlin, Padraig; Kulikowicz, Tomasz et al. (21 June 2017). "The Identification of a SIRT6 Activator from Brown Algae Fucus distichus". Marine Drugs 15 (6): 190. doi:10.3390/md15060190. PMID 28635654.
- ↑ Grabowska, Wioleta; Suszek, Małgorzata; Wnuk, Maciej et al. (28 March 2016). "Curcumin elevates sirtuin level but does not postpone in vitro senescence of human cells building the vasculature". Oncotarget 7 (15): 19201–19213. doi:10.18632/oncotarget.8450. PMID 27034011.
- ↑ Sandoval-Rodriguez, Ana; Monroy-Ramirez, Hugo Christian; Meza-Rios, Alejandra et al. (March 2020). "Pirfenidone Is an Agonistic Ligand for PPARα and Improves NASH by Activation of SIRT1/LKB1/pAMPK". Hepatology Communications 4 (3): 434–449. doi:10.1002/hep4.1474. PMID 32140659.
- ↑ 40.0 40.1 40.2 40.3 40.4 40.5 40.6 40.7 da Silva, Julie Pires (31 May 2018). Rôle de la sirtuine 1 dans la modulation des réponses apoptotique et autophagique du coeur au stress du réticulum endoplasmique (phdthesis) (in français). Université Paris Saclay (COmUE).
External links
- Sirtuins at the US National Library of Medicine Medical Subject Headings (MeSH)
 |
