Chemistry:Niacin (substance)
| |||
| Names | |||
|---|---|---|---|
| Pronunciation | /ˈnaɪəsɪn/ | ||
| Preferred IUPAC name
Pyridine-3-carboxylic acid[1] | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| 109591 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| EC Number |
| ||
| 3340 | |||
| KEGG | |||
| MeSH | Niacin | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| |||
| |||
| Properties | |||
| C6H5NO2 | |||
| Molar mass | 123.111 g·mol−1 | ||
| Appearance | White, translucent crystals | ||
| Density | 1.473 g cm−3 | ||
| Melting point | 237 °C; 458 °F; 510 K | ||
| 18 g L−1 | |||
| log P | 0.219 | ||
| Acidity (pKa) | 2.0, 4.85 | ||
| Isoelectric point | 4.75 | ||
Refractive index (nD)
|
1.4936 | ||
| 0.1271305813 D | |||
| Thermochemistry | |||
Std enthalpy of
formation (ΔfH⦵298) |
−344.9 kJ mol−1 | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−2.73083 MJ mol−1 | ||
| Pharmacology | |||
| 1=ATC code }} | C04AC01 (WHO) C10BA01 (WHO) C10AD02 (WHO) C10AD52 (WHO) | ||
| License data |
| ||
| Intramuscular, by mouth | |||
| Pharmacokinetics: | |||
| 20–45 min | |||
| Hazards | |||
| GHS pictograms | 
| ||
| GHS Signal word | Warning | ||
| H319 | |||
| P264, P280, P305+351+338, P337+313, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 193 °C (379 °F; 466 K) | ||
| 365 °C (689 °F; 638 K) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
| Clinical data | |
|---|---|
| Trade names | Niacor, Niaspan, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682518 |
| License data |
|
| Pregnancy category | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| PDB ligand | |
Template:Infobox drug/maintenance categories/container only
Niacin, also known as nicotinic acid, is an organic compound and a form of vitaminB3, an essential human nutrient.[3] It can be manufactured by plants and animals from the amino acid tryptophan.[4] Niacin is obtained in the diet from a variety of whole and processed foods, with highest contents in fortified packaged foods, meat, poultry, red fish such as tuna and salmon, lesser amounts in nuts, legumes and seeds.[3][5] Niacin as a dietary supplement is used to treat pellagra, a disease caused by niacin deficiency. Signs and symptoms of pellagra include skin and mouth lesions, anemia, headaches, and tiredness.[6] Many countries mandate its addition to wheat flour or other food grains, thereby reducing the risk of pellagra.[3][7]
The amide derivative nicotinamide (niacinamide) is a component of the coenzymes nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP+). Although niacin and nicotinamide are identical in their vitamin activity, nicotinamide does not have the same pharmacological, lipid-modifying effects or side effects as niacin, i.e., when niacin takes on the -amide group, it does not reduce cholesterol nor cause flushing.[8][9] Nicotinamide is recommended as a treatment for niacin deficiency because it can be administered in remedial amounts without causing the flushing, considered an adverse effect.[10]
Niacin is also a prescription medication.[11] Amounts far in excess of the recommended dietary intake for vitamin functions will lower blood triglycerides and low density lipoprotein cholesterol (LDL-C), and raise blood high density lipoprotein cholesterol (HDL-C, often referred to as "good" cholesterol). There are two forms: immediate-release and sustained-release niacin. Initial prescription amounts are 500 mg/day, increased over time until a therapeutic effect is achieved. Immediate-release doses can be as high as 3,000 mg/day; sustained-release as high as 2,000 mg/day.[11] Despite the proven lipid changes, niacin has not been found useful for decreasing the risk of cardiovascular disease in those already on a statin.[12] A 2010 review had concluded that niacin was effective as a mono-therapy,[13] but a 2017 review incorporating twice as many trials concluded that prescription niacin, while affecting lipid levels, did not reduce all-cause mortality, cardiovascular mortality, myocardial infarctions, nor fatal or non-fatal strokes.[14] Prescription niacin was shown to cause hepatotoxicity[15] and increase risk of type 2 diabetes.[16][17] Niacin prescriptions in the U.S. had peaked in 2009, at 9.4 million, declining to 1.3 million by 2017.[18]
Niacin has the formula C6H5NO2 and belongs to the group of the pyridinecarboxylic acids.[3] As the precursor for nicotinamide adenine dinucleotide and nicotinamide adenine dinucleotide phosphate, niacin is involved in DNA repair.[19]
Definition
Niacin is both a vitamin, i.e., an essential nutrient, marketed as a dietary supplement, and in the US, a prescription medicine. As a vitamin, it is precursor of the coenzymes nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP). These compounds are coenzymes for many dehydrogenases, participating in many hydrogen transfer processes. NAD is important in catabolism of fat, carbohydrate, protein, and alcohol, as well as cell signaling and DNA repair, and NADP mostly in anabolism reactions such as fatty acid and cholesterol synthesis.[20] Vitamin intake recommendations made by several countries are that intakes of 14–18 mg/day are sufficient to meet the needs of healthy adults.[4][21][22] Niacin or nicotinamide (niacinamide) are used for prevention and treatment of pellagra, a disease caused by lack of the vitamin.[6][20] When niacin is used as a medicine to treat elevated cholesterol and triglycerides, daily doses range from 500 to 3,000 mg/day.[23][24] High-dose nicotinamide does not have this medicinal effect.[20]
Vitamin deficiency
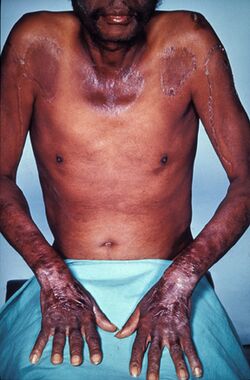
Severe deficiency of niacin in the diet causes the disease pellagra, characterized by diarrhea, sun-sensitive dermatitis involving hyperpigmentation and thickening of the skin (see image), inflammation of the mouth and tongue, delirium, dementia, and if left untreated, death.[6] Common psychiatric symptoms include irritability, poor concentration, anxiety, fatigue, loss of memory, restlessness, apathy, and depression.[20] The biochemical mechanism(s) for the observed deficiency-caused neurodegeneration are not well understood, but may rest on: A) the requirement for nicotinamide adenine dinucleotide (NAD+) to suppress the creation of neurotoxic tryptophan metabolites, B) inhibition of mitochondrial ATP generation, resulting in cell damage; C), activation of the poly (ADP-ribose) polymerase (PARP) pathway, as PARP is a nuclear enzyme involved in DNA repair, but in the absence of NAD+ can lead to cell death; D) reduced synthesis of neuro-protective brain-derived neurotrophic factor or its receptor tropomyosin receptor kinase B; or E) changes to genome expression directly due to the niacin deficiency.[25]
Niacin deficiency is rarely seen in developed countries, and it is more typically associated with poverty, malnutrition or malnutrition secondary to chronic alcoholism.[26] It also tends to occur in less developed areas where people eat maize (corn) as a staple food, as maize is the only grain low in digestible niacin. A cooking technique called nixtamalization i.e., pretreating with alkali ingredients, increases the bioavailability of niacin during maize meal/flour production.[27] For this reason, people who consume corn as tortillas or hominy are at less risk of niacin deficiency.
For treating deficiency, the World Health Organization (WHO) recommends administering niacinamide(i.e. nicotinamide) instead of niacin, to avoid the flushing side effect commonly caused by the latter. Guidelines suggest using 300 mg/day for three to four weeks.[10] Dementia and dermatitis show improvement within a week. Because deficiencies of other B-vitamins may be present, the WHO recommends a multi-vitamin in addition to the niacinamide.[10]
Hartnup disease is a hereditary nutritional disorder resulting in niacin deficiency.[28] It is named after an English family with a genetic disorder that resulted in a failure to absorb the essential amino acid tryptophan, tryptophan being a precursor for niacin synthesis. The symptoms are similar to pellagra, including red, scaly rash and sensitivity to sunlight. Oral niacin or niacinamide is given as a treatment for this condition in doses ranging from 50 to 100 mg twice a day, with a good prognosis if identified and treated early.[28] Niacin synthesis is also deficient in carcinoid syndrome, because of metabolic diversion of its precursor tryptophan to form serotonin.[3]
Measuring vitamin status
Plasma concentrations of niacin and niacin metabolites are not useful markers of niacin status.[4] Urinary excretion of the methylated metabolite N1-methyl-nicotinamide is considered reliable and sensitive. The measurement requires a 24-hour urine collection. For adults, a value of less than 5.8 μmol/day represent deficient niacin status and 5.8 to 17.5 μmol/day represents low.[4] According to the World Health Organization, an alternative mean of expressing urinary N1-methyl-nicotinamide is as mg/g creatinine in a 24-hour urine collection, with deficient defined as <0.5, low 0.5-1.59, acceptable 1.6-4.29, and high >4.3[10] Niacin deficiency occurs before the signs and symptoms of pellagra appear.[4] Erythrocyte nicotinamide adenine dinucleotide (NAD) concentrations potentially provide another sensitive indicator of niacin depletion, although definitions of deficient, low and adequate have not been established. Lastly, plasma tryptophan decreases on a low niacin diet because tryptophan converts to niacin. However, low tryptophan could also be caused by a diet low in this essential amino acid, so it is not specific to confirming vitamin status.[4]
Dietary recommendations
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The U.S. Institute of Medicine (renamed National Academy of Medicine in 2015) updated Estimated Average Requirements (EARs) and Recommended Dietary Allowances (RDAs) for niacin in 1998, also Tolerable upper intake levels (ULs). In lieu of an RDA, Adequate Intakes (AIs) are identified for populations for which there is not sufficient evidence to identify a dietary intake level that is sufficient to meet the nutrient requirements of most people.[31] (see table).
The European Food Safety Authority (EFSA) refers to the collective set of information as Dietary Reference Values (DRV), with Population Reference Intake (PRI) instead of RDA, and Average Requirement instead of EAR. For the EU, AIs and ULs have the same definition as in the US, except that units are milligrams per megajoule (MJ) of energy consumed rather than mg/day. For women (including those pregnant or lactating), men and children the PRI is 1.6 mg per megajoule. As the conversion is 1 MJ = 239 kcal, an adult consuming 2390 kilocalories should be consuming 16 mg niacin. This is comparable to US RDAs (14 mg/day for adult women, 16 mg/day for adult men).[21]
ULs are established by identifying amounts of vitamins and minerals that cause adverse effects, and then selecting as an upper limit amounts that are the "maximum daily intake unlikely to cause adverse health effects."[31] Regulatory agencies from different countries do not always agree. For the US, 30 or 35 mg for teenagers and adults, less for children.[4] The EFSA UL for adults is set at 10 mg/day - about one-third of the US value. For all of the government ULs, the term applies to niacin as a supplement consumed as one dose, and is intended as a limit to avoid the skin flush reaction. This explains why for EFSA, the recommended daily intake can be higher than the UL.[32]
Both the DRI and DRV describe amounts needed as niacin equivalents (NE), calculated as 1 mg NE = 1 mg niacin or 60 mg of the essential amino acid tryptophan. This is because the amino acid is utilized to synthesize the vitamin.[4][21]
For U.S. food and dietary supplement labeling purposes the amount in a serving is expressed as a percent of Daily Value (%DV). For niacin labeling purposes 100% of the Daily Value is 16 mg. Prior to 27 May 2016 it was 20 mg, revised to bring it into agreement with the RDA.[33][34] Compliance with the updated labeling regulations was required by 1 January 2020 for manufacturers with US$10 million or more in annual food sales, and by 1 January 2021 for manufacturers with lower volume food sales.[35][36] A table of the old and new adult daily values is provided at Reference Daily Intake.
Sources
Niacin is found in a variety of whole and processed foods, including fortified packaged foods, meat from various animal sources, seafoods, and spices.[3][37] In general, animal-sourced foods provide about 5–10 mg niacin per serving, although dairy foods and eggs have little. Some plant-sourced foods such as nuts, legumes and grains provide about 2–5 mg niacin per serving, although in some grain products this naturally present niacin is largely bound to polysaccharides and glycopeptides, making it only about 30% bioavailable. Fortified food ingredients such as wheat flour have niacin added, which is bioavailable.[5] Among whole food sources with the highest niacin content per 100 grams:
| Source[38] | Amount (mg / 100g) |
|---|---|
| Nutritional yeast[39] Serving = 2 Tbsp (16 g) contains 56 mg |
350 |
| Tuna, yellowfin | 22.1 |
| Peanuts | 14.3 |
| Peanut butter | 13.1 |
| Bacon | 10.4 |
| Tuna, light, canned | 10.1 |
| Salmon | 10.0 |
| Turkey depending on what part, how cooked | 7-12 |
| Chicken depending on what part, how cooked | 7-12 |
| Source[38] | Amount (mg / 100g) |
|---|---|
| Beef depending on what part, how cooked | 4-8 |
| Pork depending on what part, how cooked | 4-8 |
| Sunflower seeds | 7.0 |
| Tuna, white, canned | 5.8 |
| Almonds | 3.6 |
| Mushrooms, white | 3.6 |
| Cod fish | 2.5 |
| Rice, brown | 2.5 |
| Hot dogs | 2.0 |
| Source[38] | Amount (mg / 100g) |
|---|---|
| Avocado | 1.7 |
| Potato, baked, with skin | 1.4 |
| Corn (maize) | 1.0 |
| Rice, white | 0.5 |
| Kale | 0.4 |
| Eggs | 0.1 |
| Milk | 0.1 |
| Cheese | 0.1 |
| Tofu | 0.1 |
Vegetarian and vegan diets can provide adequate amounts if products such as nutritional yeast, peanuts, peanut butter, tahini, brown rice, mushrooms, avocado and sunflower seeds are included. Fortified foods and dietary supplements can also be consumed to ensure adequate intake.[5][40]
Food preparation
Niacin naturally found in food is susceptible to destruction from high heat cooking, especially in the presence of acidic foods and sauces. It is soluble in water, and so may also be lost from foods boiled in water.[41]
Food fortification
Countries fortify foods with nutrients to address known deficiencies.[7] As of 2020, 54 countries required food fortification of wheat flour with niacin or niacinamide; 14 also mandate fortification of maize flour, and 6 mandate fortification of rice.[42] From country to country, niacin fortification ranges from 1.3 to 6.0 mg/100 g.[42]
As a dietary supplement
In the United States, niacin is sold as a non-prescription dietary supplement with a range of 100 to 1000 mg per serving. These products often have a Structure/Function health claim[43] allowed by the US Food & Drug Administration (FDA). An example would be "Supports a healthy blood lipid profile." The American Heart Association strongly advises against the substitution of dietary supplement niacin for prescription niacin because of potentially serious side effects, which means that niacin should only be used under the supervision of a health care professional, and because manufacture of dietary supplement niacin is not as well-regulated by the FDA as prescription niacin.[44] More than 30 mg niacin consumed as a dietary supplement can cause skin flushing. Face, arms and chest skin turns a reddish color because of vasodilation of small subcutaneous blood vessels, accompanied by sensations of heat, tingling and itching. These signs and symptoms are typically transient, lasting minutes to hours; they are considered unpleasant rather than toxic.[5]
As lipid-modifying medication
In the United States, prescription niacin, in immediate-release and slow-release forms, is used to treat primary hyperlipidemia and hypertriglyceridemia.[23][24] It is used either as a monotherapy or in combination with other lipid-modifying drugs. Dosages start at 500 mg/day and are often gradually increased to as high as 3000 mg/day for immediate release or 2000 mg/day for slow release (also referred to as sustained release) to achieve the targeted lipid changes (lower LDL-C and triglycerides, and higher HDL-C).[23][24] Prescriptions in the US peaked in 2009, at 9.4 million and had declined to 1.3 million by 2017.[18] In late 2017, Avondale, having acquired the rights to Niacor from Upsher Smith, raised the price of the drug by more than 800%.[45]
Systematic reviews found no effect of prescription niacin on all-cause mortality, cardiovascular mortality, myocardial infarctions, nor fatal or non-fatal strokes despite raising HDL cholesterol.[12][46] Reported side effects include an increased risk of new-onset type 2 diabetes.[14][16][17][47]
Mechanisms
Niacin reduces synthesis of low-density lipoprotein cholesterol (LDL-C), very low-density lipoprotein cholesterol (VLDL-C), lipoprotein(a) and triglycerides, and increases high-density lipoprotein cholesterol (HDL-C).[48] The lipid-therapeutic effects of niacin are partly mediated through the activation of G protein-coupled receptors, including hydroxycarboxylic acid receptor 2 (HCA2)and hydroxycarboxylic acid receptor 3 (HCA3), which are highly expressed in body fat.[49][50] HCA2 and HCA3 inhibit cyclic adenosine monophosphate (cAMP) production and thus suppress the release of free fatty acids (FFAs) from body fat, reducing their availability to the liver to synthesize the blood-circulating lipids in question.[51][52][53] A decrease in free fatty acids also suppresses liver expression of apolipoprotein C3 and PPARg coactivator-1b, thus increasing VLDL-C turnover and reducing its production.[54] Niacin also directly inhibits the action of diacylglycerol O-acyltransferase 2 (DGAT2) a key enzyme for triglyceride synthesis.[53]
The mechanism behind niacin increasing HDL-C is not totally understood, but seems to occur in various ways. Niacin increases apolipoprotein A1 levels by inhibiting the breakdown of this protein, which is a component of HDL-C.[55][56] It also inhibits HDL-C hepatic uptake by suppressing production of the cholesterol ester transfer protein (CETP) gene.[48] It stimulates the ABCA1 transporter in monocytes and macrophages and upregulates peroxisome proliferator-activated receptor gamma, resulting in reverse cholesterol transport.[57]
Combined with statins
Extended release niacin was combined with the lovastatin trade-named Advicor, and with simvastatin, trade-named Simcor as prescription drug combinations. Advicor was approved by the U.S. Food and Drug Administration (FDA) in 2001.[58] Simcor was approved in 2008.[59] Subsequently, large outcome trials using these niacin and statin therapies were unable to demonstrate incremental benefit of niacin beyond statin therapy alone.[60] The FDA withdrew approval of both drugs in 2016. The reason given: "Based on the collective evidence from several large cardiovascular outcome trials, the Agency has concluded that the totality of the scientific evidence no longer supports the conclusion that a drug-induced reduction in triglyceride levels and/or increase in HDL-cholesterol levels in statin-treated patients results in a reduction in the risk of cardiovascular events." The drug company discontinued the drugs.[61]
Contraindications
Prescription immediate release (Niacor) and extended release (Niaspan) niacin are contraindicated for people with either active or a history of liver disease because both, but especially Niaspan, have been associated with instances of serious, on occasion fatal, liver failure.[24][62] Both products are contraindicated for people with existing peptic ulcer disease, or other bleeding problems because niacin lowers platelet count and interferes with blood clotting.[23][24][62] Both products are also contraindicated for women who are pregnant or expecting to become pregnant because safety during pregnancy has not been evaluated in human trials. These products are contraindicated for women who are lactating because it is known that niacin is excreted into human milk, but the amount and potential for adverse effects in the nursing infant are not known. Women are advised to either not nurse their child or discontinue the drug. High-dose niacin has not been tested or approved for use in children under 16 years.[23][24][62]
Adverse effects
The most common adverse effects of medicinal niacin (500–3000 mg) are flushing (e.g., warmth, redness, itching or tingling) of the face, neck and chest, headache, abdominal pain, diarrhea, dyspepsia, nausea, vomiting, rhinitis, pruritus and rash.[3][5][62] These can be minimized by initiating therapy at low dosages, increasing dosage gradually, and avoiding administration on an empty stomach.[62]
The acute adverse effects of high-dose niacin therapy (1–3 grams per day) – which is commonly used in the treatment of hyperlipidemias – can further include hypotension, fatigue, glucose intolerance and insulin resistance, heartburn, blurred or impaired vision, and macular edema.[3][5] With long-term use, the adverse effects of high-dose niacin therapy (750 mg per day) also include liver failure (associated with fatigue, nausea, and loss of appetite), hepatitis, and acute liver failure;[3][5] these hepatotoxic effects of niacin occur more often when extended-release dosage forms are used.[3][5] The long-term use of niacin at greater than or equal to 2 grams per day also significantly increases the risk of cerebral hemorrhage, ischemic stroke, gastrointestinal ulceration and bleeding, diabetes, dyspepsia, and diarrhea.[5]
Flushing
Flushing – a short-term dilatation of skin arterioles, causing reddish skin color – usually lasts for about 15 to 30 minutes, although sometimes can persist for weeks. Typically, the face is affected, but the reaction can extend to neck and upper chest. The cause is blood vessel dilation[3][5] due to elevation in prostaglandin GD2 (PGD2) and serotonin.[63][64][65][66] Flushing was often thought to involve histamine, but histamine has been shown not to be involved in the reaction.[63] Flushing is sometimes accompanied by a prickly or itching sensation, in particular, in areas covered by clothing.[5]
Prevention of flushing requires altering or blocking the prostaglandin-mediated pathway.[5][67] Aspirin taken half an hour before the niacin prevents flushing, as does ibuprofen. Taking niacin with meals also helps reduce this side effect.[5] Acquired tolerance will also help reduce flushing; after several weeks of a consistent dose, most people no longer experience flushing.[5] Slow- or "sustained"-release forms of niacin have been developed to lessen these side effects.[68][69]
Liver damage
Niacin in medicinal doses can cause modest elevations in serum transaminase and unconjugated bilirubin, both biomarkers of liver injury. The increases usually resolve even when drug intake is continued.[15][70][71] However, less commonly, the sustained release form of the drug can lead to serious hepatotoxicity, with onset in days to weeks. Early symptoms of serious liver damage include nausea, vomiting and abdominal pain, followed by jaundice and pruritus. The mechanism is thought to be a direct toxicity of elevated serum niacin. Lowering dose or switching to the immediate release form can resolve symptoms. In rare instances the injury is severe, and progresses to liver failure.[15]
Diabetes
The high doses of niacin used to treat hyperlipidemia have been shown to elevate fasting blood glucose in people with type 2 diabetes.[16] Long-term niacin therapy was also associated with an increase in the risk of new-onset type 2 diabetes.[16][17]
Other adverse effects
High doses of niacin can also cause niacin maculopathy, a thickening of the macula and retina, which leads to blurred vision and blindness. This maculopathy is reversible after niacin intake ceases.[72] Niaspan, the slow-release product, has been associated with a reduction in platelet content and a modest increase in prothrombin time.[24]
Pharmacology
Pharmacodynamics
Activating HCA2 has effects other than lowering serum cholesterol and triglyceride concentrations: antioxidative, anti-inflammatory, antithrombotic, improved endothelial function and plaque stability, all of which counter development and progression of atherosclerosis.[73][74]
Niacin inhibits cytochrome P450 enzymes CYP2E1, CYP2D6 and CYP3A4.[75] Niacin produces a rise in serum unconjugated bilirubin in normal individuals and in those with Gilbert's Syndrome. However, in the Gilbert's Syndrome, the rise in bilirubin is higher and clearance is delayed longer than in normal people.[76] One test used to aid in diagnosing Gilbert's Syndrome involves intravenous administration of nicotinic acid (niacin) in a dose of 50 mg over a period of 30 seconds.[70][71]
Pharmacokinetics
Both niacin and niacinamide are rapidly absorbed from the stomach and small intestine.[77] Absorption is facilitated by sodium-dependent diffusion, and at higher intakes, via passive diffusion. Unlike some other vitamins, the percent absorbed does not decrease with increasing dose, so that even at amounts of 3-4 grams, absorption is nearly complete.[20] With a one gram dose, peak plasma concentrations of 15 to 30 μg/mL are reached within 30 to 60 minutes. Approximately 88% of an oral pharmacologic dose is eliminated by the kidneys as unchanged niacin or nicotinuric acid, its primary metabolite. The plasma elimination half-life of niacin ranges from 20 to 45 minutes.[23]
Niacin and nicotinamide are both converted into the coenzyme NAD.[78] NAD converts to NADP by phosphorylation in the presence of the enzyme NAD+ kinase. High energy requirements (brain) or high turnover rate (gut, skin) organs are usually the most susceptible to their deficiency.[79] In the liver, niacinamide is converted to storage nicotinamide adenine dinucleotide (NAD). As needed, liver NAD is hydrolyzed to niacinamide and niacin for transport to tissues, there reconverted to NAD to serve as an enzyme cofactor.[20] Excess niacin is methylated in the liver to N1-methylnicotinamide (NMN) and excreted in urine as such or as the oxidized metabolite N1-methyl-2-pyridone-5-carboxamide (2-pyridone). Decreased urinary content of these metabolites is a measure of niacin deficiency.[20]
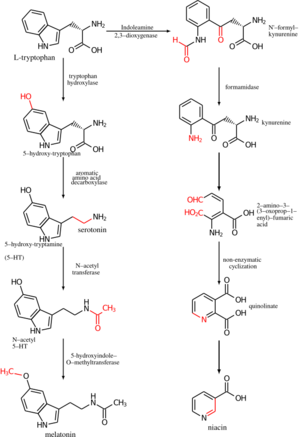
Production
Biosynthesis
In addition to absorbing niacin from diet, niacin can be synthesized from the essential amino acid tryptophan, a five-step process with the penultimate compound being quinolinic acid (see figure). Some bacteria and plants utilize aspartic acid in a pathway that also goes to quinolinic acid.[80] For humans, the efficiency of conversion is estimated as requiring 60 mg of tryptophan to make 1 mg of niacin. Riboflavin, vitamin B6 and iron are required for the process.[20] Pellagra is a consequence of a corn-dominant diet because the niacin in corn is poorly bioavailable and corn proteins are low in tryptophan compared to wheat and rice proteins.[81]
Industrial synthesis
Nicotinic acid was first synthesized in 1867 by oxidative degradation of nicotine.[82] Niacin is prepared by hydrolysis of nicotinonitrile, which, as described above, is generated by oxidation of 3-picoline. Oxidation can be effected by air, but ammoxidation is more efficient. In the latter process, nicotinonitrile is produced by ammoxidation of 3-methylpyridine. Nitrile hydratase is then used to catalyze nicotinonitrile to nicotinamide, which can be converted to niacin.[83] Alternatively, ammonia, acetic acid and paraldehyde are used to make 5-ethyl-2-methyl-pyridine, which is then oxidized to niacin.[84] New "greener" catalysts are being tested using manganese-substituted aluminophosphates that use acetyl peroxyborate as non-corrosive oxidant, avoiding producing nitrogen oxides as do traditional ammoxidations.[85]
The demand for commercial production includes for animal feed and for food fortification meant for human consumption. According to Ullmann's Encyclopedia of Industrial Chemistry, worldwide 31,000 tons of nicotinamide were sold in 2014.[82]
Chemistry
This colorless, water-soluble solid is a derivative of pyridine, with a carboxyl group (COOH) at the 3-position.[20] Other forms of vitamin B3 include the corresponding amide nicotinamide (niacinamide), where the carboxyl group has been replaced by a carboxamide group (CONH2).[20]
Preparations
Niacin is incorporated into multi-vitamin and sold as a single-ingredient dietary supplement. The latter can be immediate or slow release.[86]
Nicotinamide (niacinamide) is used to treat niacin deficiency because it does not cause the flushing adverse reaction seen with niacin. Nicotinamide may be toxic to the liver at doses exceeding 3 g/day for adults.[87]
Prescription products can be immediate release (Niacor, 500 mg tablets) or extended release (Niaspan, 500 and 1000 mg tablets). Niaspan has a film coating that delays release of the niacin, resulting in an absorption over a period of 8–12 hours. This reduces vasodilation and flushing side effects, but increases the risk of hepatotoxicity compared to the immediate release drug.[88][89]
Prescription niacin in combination with statin drugs (discontinued) is described above. A combination of niacin and laropiprant had been approved for use in Europe and marketed as Tredaptive. Laropiprant is a prostaglandin D2 binding drug shown to reduce niacin-induced vasodilation and flushing side effects.[48][90][91] A clinical trial showed no additional efficacy of Tredaptive in lowering cholesterol when used together with other statin drugs, but did show an increase in other side effects.[92] The study resulted in the withdrawal of Tredaptive from the international market.[93][94]
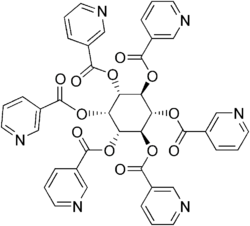
One form of dietary supplement sold in the US is inositol hexanicotinate (IHN), also called inositol nicotinate. This is inositol that has been esterified with niacin on all six of inositol's alcohol groups.[95] IHN is usually sold as "flush-free" or "no-flush" niacin in units of 250, 500, or 1000 mg/tablets or capsules. In the US, it is sold as an over-the-counter formulation, and often is marketed and labeled as niacin, thus misleading consumers into thinking they are getting an active form of the medication. While this form of niacin does not cause the flushing associated with the immediate-release products, there is not enough evidence to recommend IHN to treat hyperlipidemia.[96]
History
Niacin as a chemical compound was first described by chemist Hugo Weidel in 1873 in his studies of nicotine,[97] but that predated by many years the concept of food components other than protein, fat and carbohydrates that were essential for life. Vitamin nomenclature was initially alphabetical, with Elmer McCollum calling these fat-soluble A and water-soluble B.[98] Over time, eight chemically distinct, water-soluble B vitamins were isolated and numbered, with niacin as vitamin B3.[98]
Corn (maize) became a staple food in the southeast United States and in parts of Europe. A disease that was characterized by dermatitis of sunlight-exposed skin was described in Spain in 1735 by Gaspar Casal. He attributed the cause to poor diet.[99] In northern Italy it was named "pellagra" from the Lombard language (agra = holly-like or serum-like; pell = skin).[100][101] In time, the disease was more closely linked specifically to corn.[102] In the US, Joseph Goldberger was assigned to study pellagra by the Surgeon General of the United States. His studies confirmed a corn-based diet as the culprit, but he did not identify the root cause.[103][104]
Nicotinic acid was extracted from liver by biochemist Conrad Elvehjem in 1937. He later identified the active ingredient, referring to it as "pellagra-preventing factor" and the "anti-blacktongue factor."[105] It was also referred to as "vitamin PP", "vitamin P-P" and "PP-factor", all derived from the term "pellagra-preventive factor".[10] In the late 1930s, studies by Tom Douglas Spies, Marion Blankenhorn, and Clark Cooper confirmed that niacin cured pellagra in humans. The prevalence of the disease was greatly reduced as a result.[106]
In 1942, when flour enrichment with nicotinic acid began, a headline in the popular press said "Tobacco in Your Bread." In response, the Council on Foods and Nutrition of the American Medical Association approved of the Food and Nutrition Board's new names niacin and niacin amide for use primarily by non-scientists. It was thought appropriate to choose a name to dissociate nicotinic acid from nicotine, to avoid the perception that vitamins or niacin-rich food contains nicotine, or that cigarettes contain vitamins. The resulting name niacin was derived from nicotinic acid + vitamin.[107][108]
Carpenter found in 1951, that niacin in corn is biologically unavailable, and can be released only in very alkaline lime water of pH 11. This explains why a Latin-American culture that used alkali-treated cornmeal to make tortilla was not at risk for niacin deficiency.[109]
In 1955, Altschul and colleagues described large amounts of niacin as having a lipid-lowering property.[110] As such, niacin is the oldest known lipid-lowering drug.[111] Lovastatin, the first 'statin' drug, was first marketed in 1987.[112]
Research
In animal models and in vitro, niacin produces marked anti-inflammatory effects in a variety of tissues – including the brain, gastrointestinal tract, skin, and vascular tissue – through the activation of hydroxycarboxylic acid receptor 2 (HCA2), also known as niacin receptor 1 (NIACR1).[113][114][115][116] Unlike niacin, nicotinamide does not activate NIACR1; however, both niacin and nicotinamide activate the G protein-coupled estrogen receptor (GPER) in vitro.[117]
References
- ↑ "Chapter P-6. Applications to Specific Classes of Compounds". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: Royal Society of Chemistry. 2014. pp. 648–1047. doi:10.1039/9781849733069-00648. ISBN 978-0-85404-182-4.
- ↑ 2.0 2.1 "Niacin Use During Pregnancy". 29 July 2019. https://www.drugs.com/pregnancy/niacin.html.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 "Niacin". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, OR. 8 October 2018. https://lpi.oregonstate.edu/mic/vitamins/niacin.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 Institute of Medicine (1998). "Niacin". Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: The National Academies Press. pp. 123–149. ISBN 978-0-309-06554-2. https://www.nap.edu/read/6015/chapter/8. Retrieved 29 August 2018.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 "Niacin Fact Sheet for Health Professionals". Office of Dietary Supplements, US National Institutes of Health. 3 June 2020. https://ods.od.nih.gov/factsheets/Niacin-HealthProfessional/.
- ↑ 6.0 6.1 6.2 "Pellagra: dermatitis, dementia, and diarrhea". International Journal of Dermatology 43 (1): 1–5. January 2004. doi:10.1111/j.1365-4632.2004.01959.x. PMID 14693013.
- ↑ 7.0 7.1 "Why fortify?". Food Fortification Initiative. 2017. http://www.ffinetwork.org/why_fortify/index.html.
- ↑ "Niacin versus niacinamide". CMAJ 147 (7): 990. October 1992. PMID 1393911.
- ↑ "Niacin requirements for genomic stability". Mutation Research 733 (1–2): 14–20. May 2012. doi:10.1016/j.mrfmmm.2011.11.008. PMID 22138132. https://zenodo.org/record/1143032.
- ↑ 10.0 10.1 10.2 10.3 10.4 Pellagra And Its Prevention And Control In Major Emergencies (Report). World Health Organization (WHO). 2000. WHO/NHD/00.10.
- ↑ 11.0 11.1 "Niacin". 16 March 2019. https://www.drugs.com/niacin.html.
- ↑ 12.0 12.1 "Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients". BMJ 349: g4379. July 2014. doi:10.1136/bmj.g4379. PMID 25038074.
- ↑ "Meta-analysis of the effect of nicotinic acid alone or in combination on cardiovascular events and atherosclerosis". Atherosclerosis 210 (2): 353–61. June 2010. doi:10.1016/j.atherosclerosis.2009.12.023. PMID 20079494.
- ↑ 14.0 14.1 "Niacin for primary and secondary prevention of cardiovascular events". The Cochrane Database of Systematic Reviews 2017 (6): CD009744. June 2017. doi:10.1002/14651858.CD009744.pub2. PMID 28616955.
- ↑ 15.0 15.1 15.2 "Niacin". IN: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury (Internet). Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases. February 2014.
- ↑ 16.0 16.1 16.2 16.3 "Cardiovascular drugs that increase the risk of new-onset diabetes". Am. Heart J. 167 (4): 421–8. April 2014. doi:10.1016/j.ahj.2013.12.025. PMID 24655688. http://www.escholarship.org/uc/item/6gd606b1.
- ↑ 17.0 17.1 17.2 "Niacin therapy and the risk of new-onset diabetes: a meta-analysis of randomised controlled trials". Heart 102 (3): 198–203. February 2016. doi:10.1136/heartjnl-2015-308055. PMID 26370223.
- ↑ 18.0 18.1 "Niacin - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Niacin.
- ↑ "B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review". Nutrients 8 (2): 68. January 2016. doi:10.3390/nu8020068. PMID 26828517.
- ↑ 20.00 20.01 20.02 20.03 20.04 20.05 20.06 20.07 20.08 20.09 BP Marriott, ed (2020). "Niacin". Present Knowledge in Nutrition, Eleventh Edition. London, United Kingdom: Academic Press (Elsevier). pp. 209–24. ISBN 978-0-323-66162-1.
- ↑ 21.0 21.1 21.2 "Overview on Dietary Reference Values for the EU population as derived by the EFSA Panel on Dietetic Products, Nutrition and Allergies". 2017. https://www.efsa.europa.eu/sites/default/files/assets/DRV_Summary_tables_jan_17.pdf.
- ↑ 22.0 22.1 22.2 "Nutrient reference values for Australia and New Zealand". National Health and Medical Research Council. 9 September 2005. http://www.nhmrc.gov.au/_files_nhmrc/file/publications/synopses/n35.pdf.
- ↑ 23.0 23.1 23.2 23.3 23.4 23.5 "NIACOR-niacin tablet". March 2020. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ce739d68-d89c-437c-90fb-3c0c45140f22##.
- ↑ 24.0 24.1 24.2 24.3 24.4 24.5 24.6 "Niaspan Patient Package and Product Information (PPPI)". December 2018. http://www.rxabbott.com/pdf/niaspan.pdf.
- ↑ "The biochemical pathways of central nervous system neural degeneration in niacin deficiency". Neural Regeneration Research 9 (16): 1509–13. August 2014. doi:10.4103/1673-5374.139475. PMID 25317166.
- ↑ "Pellagra encephalopathy following B-complex vitamin treatment without niacin". International Journal of Psychiatry in Medicine 34 (1): 91–5. March 2004. doi:10.2190/29XV-1GG1-U17K-RGJH. PMID 15242145. http://baywood.metapress.com/link.asp?id=29xv1gg1u17krgjh. Retrieved 27 November 2009.
- ↑ "Effect of processing on distribution and in vitro availability of niacin of corn (Zea mays)". Food Technol 15: 450–4. 1961.
- ↑ 28.0 28.1 "Hartnup Disease". January 2020. https://www.merckmanuals.com/professional/pediatrics/congenital-renal-transport-abnormalities/hartnup-disease?query=Hartnup%20Disease.
- ↑ 29.0 29.1 Health Canada (2005-07-20). "Dietary Reference Intakes". Government of Canada. https://www.canada.ca/en/health-canada/services/food-nutrition/healthy-eating/dietary-reference-intakes/tables/reference-values-vitamins-dietary-reference-intakes-tables-2005.html.
- ↑ 30.0 30.1 30.2 "Tolerable Upper Intake Levels for Vitamins and Minerals". European Food Safety Authority. February 2006. http://www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/ndatolerableuil.pdf.
- ↑ 31.0 31.1 "Nutrient Recommendations: Dietary Reference Intakes (DRI)". https://ods.od.nih.gov/Health_Information/Dietary_Reference_Intakes.aspx.
- ↑ "Tolerable Upper Intake Levels For Vitamins And Minerals". European Food Safety Authority. 2006. http://www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/ndatolerableuil.pdf.
- ↑ "Federal Register May 27, 2016 Food Labeling: Revision of the Nutrition and Supplement Facts Labels". https://www.gpo.gov/fdsys/pkg/FR-2016-05-27/pdf/2016-11867.pdf.
- ↑ "Daily Value Reference of the Dietary Supplement Label Database (DSLD)". https://www.dsld.nlm.nih.gov/dsld/dailyvalue.jsp.
- ↑ "Changes to the Nutrition Facts Label". 27 May 2016. https://www.fda.gov/food/food-labeling-nutrition/changes-nutrition-facts-label.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Industry Resources on the Changes to the Nutrition Facts Label". 21 December 2018. https://www.fda.gov/food/food-labeling-nutrition/industry-resources-changes-nutrition-facts-label.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Niacin content per 100 grams; select food subset, abridged list by food groups". United States Department of Agriculture, Agricultural Research Service, USDA Branded Food Products Database v.3.6.4.1. 17 January 2017. https://ndb.nal.usda.gov/ndb/nutrients/report/nutrientsfrm?max=25&offset=0&totCount=0&nutrient1=406&nutrient2=&nutrient3=&subset=1&fg=13&fg=1&fg=15&fg=17&fg=10&fg=5&fg=2&fg=11&sort=c&measureby=g.
- ↑ 38.0 38.1 38.2 "USDA National Nutrient Database for Standard Reference Legacy: Niacin". 2018. https://www.nal.usda.gov/sites/www.nal.usda.gov/files/niacin.pdf.
- ↑ "Nutritional Yeast Flakes (two tablespoons = 16 grams". https://nutritiondata.self.com/facts/custom/1323565/2.
- ↑ "Vitamin B3 (Niacin)". 2000. https://www.vivahealth.org.uk/a-z/vitamin-b3-niacin.
- ↑ "Effects of Cooking on Vitamins (Table)". Beyondveg. http://www.beyondveg.com/tu-j-l/raw-cooked/raw-cooked-2e.shtml.
- ↑ 42.0 42.1 "Map: Count of Nutrients In Fortification Standards". https://fortificationdata.org/map-number-of-nutrients/.
- ↑ "Structure/Function Claims". December 2017. https://www.fda.gov/food/food-labeling-nutrition/structurefunction-claims.
- ↑ "Cholesterol Medications". 10 November 2018. https://www.heart.org/en/health-topics/cholesterol/prevention-and-treatment-of-high-cholesterol-hyperlipidemia/cholesterol-medications.
- ↑ "US drugmaker raises price of vitamins by more than 800%". 10 December 2017. https://www.ft.com/content/477521fa-dc34-11e7-a039-c64b1c09b482.
- ↑ "Niacin Therapy, HDL Cholesterol, and Cardiovascular Disease: Is the HDL Hypothesis Defunct?". Curr Atheroscler Rep 17 (8): 43. August 2015. doi:10.1007/s11883-015-0521-x. PMID 26048725.
- ↑ "Role of Niacin in Current Clinical Practice: A Systematic Review". The American Journal of Medicine 130 (2): 173–187. February 2017. doi:10.1016/j.amjmed.2016.07.038. PMID 27793642.
- ↑ 48.0 48.1 48.2 "Niacin: the evidence, clinical use, and future directions". Current Atherosclerosis Reports 14 (1): 49–59. February 2012. doi:10.1007/s11883-011-0212-1. PMID 22037771.
- ↑ "Molecular identification of nicotinic acid receptor". Biochemical and Biophysical Research Communications 303 (1): 364–9. March 2003. doi:10.1016/S0006-291X(03)00342-5. PMID 12646212.
- ↑ "Molecular identification of high and low affinity receptors for nicotinic acid". The Journal of Biological Chemistry 278 (11): 9869–74. March 2003. doi:10.1074/jbc.M210695200. PMID 12522134.
- ↑ "Nicotinic acid: pharmacological effects and mechanisms of action". Annual Review of Pharmacology and Toxicology 48 (1): 79–106. 2008. doi:10.1146/annurev.pharmtox.48.113006.094746. PMID 17705685.
- ↑ "Future of GPR109A agonists in the treatment of dyslipidaemia". Diabetes, Obesity & Metabolism 13 (8): 685–91. August 2011. doi:10.1111/j.1463-1326.2011.01400.x. PMID 21418500.
- ↑ 53.0 53.1 "Molecular pathways and agents for lowering LDL-cholesterol in addition to statins". Pharmacol Ther 126 (3): 263–78. June 2010. doi:10.1016/j.pharmthera.2010.02.006. PMID 20227438.
- ↑ "Regulation of hepatic ApoC3 expression by PGC-1β mediates hypolipidemic effect of nicotinic acid". Cell Metabolism 12 (4): 411–9. October 2010. doi:10.1016/j.cmet.2010.09.001. PMID 20889132.
- ↑ "Niacin, lipids, and heart disease". Curr Cardiol Rep 5 (6): 470–6. November 2003. doi:10.1007/s11886-003-0109-x. PMID 14558989.
- ↑ "Niacin: another look at an underutilized lipid-lowering medication". Nature Reviews. Endocrinology 8 (9): 517–28. September 2012. doi:10.1038/nrendo.2012.22. PMID 22349076.
- ↑ "Stimulation of CD36 and the key effector of reverse cholesterol transport ATP-binding cassette A1 in monocytoid cells by niacin". Biochemical Pharmacology 67 (3): 411–9. February 2004. doi:10.1016/j.bcp.2003.09.014. PMID 15037193.
- ↑ "Advicor (Niacin Extended-Release & Lovastatin) Tablets". 13 September 2002. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/21-249_Advicor.cfm.
- ↑ "Drugs.com, Abbott Receives FDA Approval for Simcor (Niaspan / simvastatin), a Novel Combination Medicine for Comprehensive Cholesterol Management". https://www.drugs.com/newdrugs/abbott-receives-fda-approval-simcor-niaspan-simvastatin-novel-combination-medicine-comprehensive-846.html.
- ↑ "Is HPS2-THRIVE the death knell for niacin?". J Clin Lipidol 9 (3): 343–50. 2015. doi:10.1016/j.jacl.2015.01.008. PMID 26073392.
- ↑ "AbbVie Inc.; Withdrawal of Approval of New Drug Applications for ADVICOR and SIMCOR". 18 April 2016. https://www.federalregister.gov/documents/2016/04/18/2016-08894/abbvie-inc-withdrawal-of-approval-of-new-drug-applications-for-advicor-and-simcor.
- ↑ 62.0 62.1 62.2 62.3 62.4 Niaspan (niacin extended-release) tablets prescribing information. AbbVie Inc., US-NIAS-180036, North Chicago, IL 60064, December 2018
- ↑ 63.0 63.1 "Niacin-induced "flush" involves release of prostaglandin D2 from mast cells and serotonin from platelets: evidence from human cells in vitro and an animal model". The Journal of Pharmacology and Experimental Therapeutics 327 (3): 665–72. December 2008. doi:10.1124/jpet.108.141333. PMID 18784348.
- ↑ "GPR109A (PUMA-G/HM74A) mediates nicotinic acid-induced flushing". The Journal of Clinical Investigation 115 (12): 3634–40. December 2005. doi:10.1172/JCI23626. PMID 16322797.
- ↑ "Nicotinic acid-induced flushing is mediated by activation of epidermal langerhans cells". Molecular Pharmacology 70 (6): 1844–9. December 2006. doi:10.1124/mol.106.030833. PMID 17008386.
- ↑ "Langerhans cells release prostaglandin D2 in response to nicotinic acid". The Journal of Investigative Dermatology 126 (12): 2637–46. December 2006. doi:10.1038/sj.jid.5700586. PMID 17008871.
- ↑ "Mechanism of action of niacin". The American Journal of Cardiology 101 (8A): 20B–26B. April 2008. doi:10.1016/j.amjcard.2008.02.029. PMID 18375237.
- ↑ Katzung, Bertram G. (2006). Basic and clinical pharmacology. New York: McGraw-Hill Medical Publishing Division. ISBN 978-0-07-145153-6.
- ↑ "Options for therapeutic intervention: How effective are the different agents?". European Heart Journal Supplements 8 (F): F47–F53. October 2006. doi:10.1093/eurheartj/sul041.
- ↑ 70.0 70.1 "The Nicotinic Acid Provocation Test and Unconjugated Hyperbilirubinaemia". The Ulster Medical Journal 60 (1): 49–52. April 1991. PMID 1853497.
- ↑ 71.0 71.1 "Nicotinic Acid Test in the Diagnosis of Gilbert's Syndrome: Correlation With Bilirubin Clearance". Gut 22 (8): 663–668. August 1981. doi:10.1136/gut.22.8.663. PMID 7286783.
- ↑ "Ocular Effects of Niacin: A Review of the Literature". Med Hypothesis Discov Innov Ophthalmol 4 (2): 64–71. 2015. PMID 26060832.
- ↑ "Niacin in the Treatment of Hyperlipidemias in Light of New Clinical Trials: Has Niacin Lost its Place?". Med. Sci. Monit. 21: 2156–62. July 2015. doi:10.12659/MSM.893619. PMID 26210594.
- ↑ "Evidence that niacin inhibits acute vascular inflammation and improves endothelial dysfunction independent of changes in plasma lipids". Arteriosclerosis, Thrombosis, and Vascular Biology 30 (5): 968–75. May 2010. doi:10.1161/ATVBAHA.109.201129. PMID 20167660.
- ↑ "Inhibition of human P450 enzymes by nicotinic acid and nicotinamide". Biochemical and Biophysical Research Communications 317 (3): 950–6. May 2004. doi:10.1016/j.bbrc.2004.03.137. PMID 15081432.
- ↑ Nathan and Oski's Hematology of Infancy and Childhood. January 2009. pp. 118–119. ISBN 9781416034308.
- ↑ "Intestinal absorption of water-soluble vitamins in health and disease". The Biochemical Journal 437 (3): 357–372. August 2011. doi:10.1042/BJ20110326. PMID 21749321.
- ↑ Lehninger principles of biochemistry. New York: Worth Publishers. 2000. ISBN 978-1-57259-153-0. https://archive.org/details/lehningerprincip01lehn.
- ↑ "Pellagra among chronic alcoholics: clinical and pathological study of 20 necropsy cases". Journal of Neurology, Neurosurgery, and Psychiatry 44 (3): 209–15. March 1981. doi:10.1136/jnnp.44.3.209. PMID 7229643.
- ↑ "Nicotinamide adenine dinucleotide biosynthesis and pyridine nucleotide cycle metabolism in microbial systems". Microbiol. Rev. 44 (1): 83–105. 1 March 1980. doi:10.1128/MMBR.44.1.83-105.1980. PMID 6997723.
- ↑ "The Relationship of Pellagra to Corn and the Low Availability of Niacin in Cereals". Nutritional Adequacy, Nutrient Availability and Needs. Experientia Supplementum. 44. 1983. pp. 197–222. doi:10.1007/978-3-0348-6540-1_12. ISBN 978-3-0348-6542-5.
- ↑ 82.0 82.1 "Vitamins, 11. Niacin (Nicotinic Acid, Nicotinamide". Ullmann's Encyclopedia of Industrial Chemistry (6th ed.). Weinheim: Wiley-VCH. 2015. pp. 1–9. doi:10.1002/14356007.o27_o14.pub2. ISBN 978-3-527-30385-4.
- ↑ "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. 2007. doi:10.1002/14356007.a22_399.
- ↑ "100 Years of Progress with LONZA". Chimia 51 (6): 259–69. June 1997. doi:10.2533/chimia.1997.259. https://www.ingentaconnect.com/content/scs/chimia/1997/00000051/00000006/art00002?crawler=true&mimetype=application/pdf. Retrieved 8 October 2020.
- ↑ Sarah Everts (2008). "Clean Catalysis: Environmentally friendly synthesis of niacin generates less inorganic waste". Chemical & Engineering News. ISSN 0009-2347.
- ↑ "A systematic review on evidence of the effectiveness and safety of a wax-matrix niacin formulation". Journal of Clinical Lipidology 6 (2): 121–31. 1 March 2012. doi:10.1016/j.jacl.2011.07.003. PMID 22385545.
- ↑ "Safety of high-dose nicotinamide: a review". Diabetologia 43 (11): 1337–45. November 2000. doi:10.1007/s001250051536. PMID 11126400.
- ↑ "A case for immediate-release niacin". Heart & Lung 41 (1): 95–8. 2012. doi:10.1016/j.hrtlng.2010.07.019. PMID 21414665.
- ↑ "Pharmacokinetics and dose recommendations of Niaspan in chronic kidney disease and dialysis patients". Nephrology, Dialysis, Transplantation 26 (1): 276–82. January 2011. doi:10.1093/ndt/gfq344. PMID 20562093.
- ↑ "Suppression of niacin-induced vasodilation with an antagonist to prostaglandin D2 receptor subtype 1". Clinical Pharmacology and Therapeutics 81 (6): 849–57. June 2007. doi:10.1038/sj.clpt.6100180. PMID 17392721.
- ↑ "Extended-release niacin/laropiprant: reducing niacin-induced flushing to better realize the benefit of niacin in improving cardiovascular risk factors". Cardiology Clinics 26 (4): 547–60. November 2008. doi:10.1016/j.ccl.2008.06.007. PMID 19031552.
- ↑ "Effects of extended-release niacin with laropiprant in high-risk patients". N. Engl. J. Med. 371 (3): 203–12. July 2014. doi:10.1056/NEJMoa1300955. PMID 25014686. http://www.nejm.org/doi/pdf/10.1056/NEJMoa1300955.
- ↑ "Niacin/Laropiprant Products to Be Suspended Worldwide". 11 January 2013. http://www.medscape.com/viewarticle/777519.
- ↑ "Merck begins overseas recall of HDL cholesterol drug". Reuters. 11 January 2013. https://www.reuters.com/article/us-merck-cholesteroldrug-withdrawal-idUSBRE90A0MB20130111?feedType=RSS&feedName=healthNews.
- ↑ "Inositol hexanicotinate (inositol hexaniacinate) as a source of niacin (vitamin B3) added for nutritional purposes in food supplements". The EFSA Journal 949: 1–20. January 2009. https://www.efsa.europa.eu/en/efsajournal/pub/949.
- ↑ "No-Flush Niacin for the Treatment of Hyperlipidemia". 15 January 2003. http://www.medscape.com/viewarticle/447528.
- ↑ "Zur Kenntniss des Nicotins". Justus Liebigs Annalen der Chemie und Pharmacie 165 (2): 330–49. 1873. doi:10.1002/jlac.18731650212. https://zenodo.org/record/1427317.
- ↑ 98.0 98.1 The Vitamins: Fundamental Aspects in Nutrition and Health (3rd ed.). Elsevier, Boston, MA. 2007. pp. 7–33. ISBN 978-0-080-56130-1. https://books.google.com/books?id=1CMHiWum0Y4C&pg=PA16.
- ↑ "The natural and medical history of the principality of the Asturias". Classic Descriptions of Disease (3rd ed.). Springfield: Charles C Thomas. 1945. pp. 607–12.
- ↑ F. Cherubini, Vocabolario Milanese-Italiano, Imp. Regia Stamperia, 1840-43, vol. I, III.
- ↑ "Definition of Pellagra". MedicineNet.com. http://www.medterms.com/script/main/art.asp?articlekey=4821.
- ↑ Cesare Lombroso, Studi clinici ed esperimentali sulla natura, causa e terapia delle pellagra (Bologna: Fava e Garagnani, 1869)
- ↑ "Joseph Goldberger: an unsung hero of American clinical epidemiology". Ann Intern Med 121 (5): 372–75. September 1994. doi:10.7326/0003-4819-121-5-199409010-00010. PMID 8042827.
- ↑ "Dr. Joseph Goldberger and the War on Pellagra | Ashes on the Potomac". https://history.nih.gov/pages/viewpage.action?pageId=8883184.
- ↑ "The isolation and identification of the anti-blacktongue factor J". J. Biol. Chem. 123 (1): 137–49. 1938. doi:10.1016/S0021-9258(18)74164-1. http://www.jbc.org/content/123/1/137.full.pdf.
- ↑ Ruth Hanna Sachs, White Rose History. Volume I. 2003. Appendix D, p. 2 ISBN:978-0-9710541-9-6 "Men of the Year, outstanding in comprehensive science were three medical researchers who discovered that nicotinic acid was a cure for human pellagra: Drs. Tom Douglas Spies of Cincinnati General Hospital, Marion Arthur Blankenhorn of the University of Cincinnati, Clark Niel Cooper of Waterloo, Iowa."
- ↑ "Niacin and Niacin Amide". Journal of the American Medical Association 118 (10): 819. 7 March 1942. doi:10.1001/jama.1942.02830100049011.
- ↑ "Niacin and Nicotinic Acid". Journal of the American Medical Association 118 (10): 823. 7 March 1942. doi:10.1001/jama.1942.02830100053014.
- ↑ "Raw versus processed corn in niacin-deficient diets". The Journal of Nutrition 45 (1): 21–8. September 1951. doi:10.1093/jn/45.1.21. PMID 14880960.
- ↑ "Influence of nicotinic acid on serum cholesterol in man". Archives of Biochemistry and Biophysics 54 (2): 558–9. February 1955. doi:10.1016/0003-9861(55)90070-9. PMID 14350806.
- ↑ "Niacin: an old lipid drug in a new NAD+ dress". J. Lipid Res. 60 (4): 741–6. April 2019. doi:10.1194/jlr.S092007. PMID 30782960.
- ↑ "The $10 billion pill". Fortune 147 (1): 58–62, 66, 68. January 2003. PMID 12602122. http://archive.fortune.com/magazines/fortune/fortune_archive/2003/01/20/335643/index.htm.
- ↑ "Nutritional or pharmacological activation of HCA(2) ameliorates neuroinflammation". Trends in Molecular Medicine 21 (4): 245–55. April 2015. doi:10.1016/j.molmed.2015.02.002. PMID 25766751.
- ↑ "GPR109A and vascular inflammation". Current Atherosclerosis Reports 15 (5): 325. May 2013. doi:10.1007/s11883-013-0325-9. PMID 23526298.
- ↑ "Anti-inflammatory effects of the hydroxycarboxylic acid receptor 2". Metabolism 65 (2): 102–13. February 2016. doi:10.1016/j.metabol.2015.10.001. PMID 26773933.
- ↑ "A novel treatment target for Parkinson's disease". Journal of the Neurological Sciences 347 (1–2): 34–8. December 2014. doi:10.1016/j.jns.2014.10.024. PMID 25455298.
- ↑ "Niacin activates the G protein estrogen receptor (GPER)-mediated signalling". Cellular Signalling 26 (7): 1466–75. July 2014. doi:10.1016/j.cellsig.2014.03.011. PMID 24662263.
External links
- "Niacin". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/niacin.